Difference between revisions of "Laboratory Techniques"
| Line 393: | Line 393: | ||
|- | |- | ||
|} | |} | ||
| + | |||
| + | '''Yeast Growth Media''' | ||
| + | |||
| + | This is a simple non-synthetic yeast growth media shared by Cory Widmayer on Milk The Funk, and is based off of [https://academic.oup.com/femsyr/article/13/1/34/544881 Leite et al. (2013)]. See [https://www.facebook.com/groups/MilkTheFunk/permalink/3328794017148789/ Widmayer's post on MTF] for directions on using this media (requires aeration). This media and method are claimed to grow ''Brettanomyces'' very quickly. Widmayer also provides a synthetic version of the recipe on the MTF post. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | Glucose or another sugar type || 100 grams | ||
| + | |- | ||
| + | | Yeast Extract || 90 grams | ||
| + | |- | ||
| + | | Distilled Water || Fill to 1000 mL | ||
| + | |- | ||
| + | } | ||
====Misc==== | ====Misc==== | ||
| − | |||
* [https://www.sciencedirect.com/science/article/abs/pii/S0168160519303460 A simple procedure for detecting ''Brettanomyces bruxellensis'' in wine environment by RNA-FISH using a novel probe.] | * [https://www.sciencedirect.com/science/article/abs/pii/S0168160519303460 A simple procedure for detecting ''Brettanomyces bruxellensis'' in wine environment by RNA-FISH using a novel probe.] | ||
Revision as of 21:08, 9 March 2020
This focuses on home lab and small brewery lab techniques.
Contents
Equipment
General (links to other pages)
- Microscope
- How To Use a Microscope
- pH Meter
- MTF thread on advice for what kind of pipette to get for cell counting.
Bunsen Burner
Density Meter
Density Meter's are a much more accurate tool for testing things like ethanol content as well as other things that can be useful in a brewery setting.
Dissolved Oxygen Meter
Dissolved oxygen meters play a large role in brewery QC of the final packaged products as well as oxygenation of wort pre-fermentation. Although cheaper DO meters can be used to find the PPM(parts per million) of oxygen in the wort pre-fermentation, more expensive advanced equipment is needed post fermentation as you'll need equipment that is capable of reading much lower levels of DO on a ppb level. When monitoring DO its crucial to be under 100 ppb packaged DO and for hop driven beers its ideal to be under 50 ppb or else you risk oxidation and other off flavors associated with oxygen.
During DO packaging testing, Justin Amaral found that breweries have issues with DO coming directly from their canning/bottling system. The most common factor found in high DO readings was in proper purging of lines when starting up the machines or taking a break as well as mechanical failure.
Orbital Shaker
An orbital shaker is a laboratory device used for mixing substances or maintaining movement of fluids. Maintaining movement of liquids has been shown to help some microorganisms grow. For example, running a shaker at 80 RPM for Brettanomyces starters is an effective way to grow this genra (see Brettanomyces starters) [1]. For a home orbital shaker example, see Example of a Home Lab Orbital Shaker.
PCR/qPCR
https://www.minipcr.com/product/minipcr-dna-discovery-system/
https://www.facebook.com/groups/MilkTheFunk/permalink/1888017211226484/ - Under Joe I's comment
https://www.weberscientific.com/beer-spoilage-micro-test-kit-microbiologique (see this MTF thread).
https://www.facebook.com/groups/MilkTheFunk/permalink/2069735766387960/
S. cerevisiae var. diastaticus
- MTF thread on identifying Saccharomyces cerevisiae var. diastaticus using "Instagene". Richard Preiss warns of false positives when using STA1 PCR.
- See also: S. cerevisiae var. diastaticus.
Titratable Acidity Meter
TA meters can be a very useful tool in sour beer brewing. Although its generally been used in the wine world to measure the acidity of wines, it can be used on beer as well. Although pH can give you rough look how acidic something is, it doesn't really give you a accurate outlook on how acidic something is on the palate. Please refer to http://www.milkthefunk.com/wiki/Titratable_Acidity for more info.
UV/VIS Spectrophotometer
A Spectrophotometer can be used for an array of tests. They are commonly used to analyze SRM, IBU, ethanol content and other wort compositions. They can also be used for cell counts as well as other microbiology facets.
UV Plate Cooling Cabinet
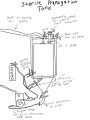
Using a UV sterilization cabinet can not only help cool/sterilize freshly poured plates but it can be used to UV sterilize other things as well. The build itself is quite simple. You will have to do some basic wiring.
Ideally you'll want a Laminar flow hood with a UV light in the hood, but this will do for just cooling plates or doing quick UV sterilization.
Parts List -
Light Ballast https://www.amazon.com/gp/product/B00AB32J7S/ref=oh_aui_detailpage_o04_s01?ie=UTF8&psc=1 Lamp Mount https://www.amazon.com/gp/product/B0036ZA966/ref=oh_aui_detailpage_o04_s01?ie=UTF8&psc=1 4 pin connector https://www.amazon.com/gp/product/B003B92NP2/ref=oh_aui_detailpage_o04_s01?ie=UTF8&psc=1 UV Light https://www.amazon.com/gp/product/B001HB3E2W/ref=oh_aui_detailpage_o06_s00?ie=UTF8&psc=1 Plexiglass https://www.amazon.com/gp/product/B019D0DUDQ/ref=oh_aui_detailpage_o03_s00?ie=UTF8&psc=1
Growth Media
General Notes
Richard Preiss from Escarpment Laboratories reported better agar results when limiting autoclave times to 15 minutes for recipes that include copper-containing ingredients [2].
See also:
- MTF tips on autoclaving wort extract based media in such a way that prevents precipitation and darkening.
- MTF tips on what media to use for wild yeast isolation.
- For techniques on separating yeast strains from themselves and from bacteria, see Yeast/Bacteria Isolation.
- To detect Brettanomyces in a Saccharomyces culture, WLN (or WLN along with DBDM) or even something as cheap as YPD can be used because Saccharomyces will show significant colony growth at 3 days, while Brettanomyces colonies will grow at 5-10 days [3].
Reference on DBDM: https://www.facebook.com/groups/MilkTheFunk/permalink/1805210829507123/?comment_id=1805397166155156&comment_tracking=%7B%22tn%22%3A%22R%22%7D
Lactobacillus/Pediococcus
See Rogosa SL Agar.
MRS Media
| Chemical | Usage Amount |
|---|---|
| Dextrose | 20 grams |
| Peptone | 10 grams |
| Beef Extract | 8 grams |
| Yeast Extract | 4 grams |
| Sodium Acetate | 5 grams |
| Dipotassium Hydrogen Phosphate | 2 grams |
| Ammonium Citrate | 2 grams |
| Manganous Sulfate Tetrahydrate | 0.05 grams |
| Magnesium Sulfate Heptahydrate | 0.2 grams |
| Distilled/De-ionized Water | Fill to 1000 ML |
Catalase enzyme can be spread onto MRS media to assist with culturing so-called "viable but non-culturable" (VBNC) bacteria cells; see the Quality Assurance page for details.
NBB Media
NBB media can also be used to help detect/isolate spoilage bacteria. This is a pre-made media by Doehler. More info can be found at NBB Media. Although ASBC suggests this media its most likely favored by them as their recommendations are generally products sold by Siebel. After a quick discussion with Richard Preiss cheaper media can be used to reach the same goals. Using HLP, WLN w/ cycloheximide and tween 80, and MRS w/ cycloheximide and tween 80 you can achieve the same results as using the array of NBB media available.
ABD Media
Advanced beer-spoiler detection medium (ABD), which is basically MRS with some of the MRS substituted for beer, has reportedly been shown to be a better growth medium for beer-spoiler LAB which have adapted to the brewing environment and are difficult to grow on other media. This media also has the advantage of inhibiting the growth of other microorganisms that are not beer spoilers. In order to grow some very slow growing strains, microcolony methods using carboxyfluorescein diacetate (CFDA) and species-specific fluorescence in situ hybridization (FISH) allows detection of slow-growing strains of LAB within 3 days, although the CFDA and FISH approaches require special equipment that might not be available for some QC laboratories [4][5].
| Chemical | Usage Amount |
|---|---|
| MRS broth (powder) | 2.61 grams |
| Sodium acetate | 0.5 grams |
| Clycloheximide | 10 milligrams |
| Agar | 15 grams |
| Beer (presumably lager beer with low IBU) | 1000 milliliters |
| Final pH | 5.0 |
Saccharomyces
A wide variety of media can be used for Saccharomyces. Bromocresol Green can also be added to these media, as in the commercial WLN formulation. Most Saccharomyces cannot metabolize this dye, causing the colonies to stain green. Chloramphenicol can also be added to eliminate bacterial growth [6]. Although not all of these media are specifically for your average brewers Saccharomyces, most strains should have no issues growing.
YPD Media
| Chemical | Usage Amount |
|---|---|
| Yeast Extract | 10 grams |
| Peptone | 20 grams |
| Dextrose | 20 grams |
| Agar(optional) | 15 grams |
| Distilled Water | Fill to 1000 ML |
MYPG Media
| Chemical | Usage Amount |
|---|---|
| Malt Extract | 3 grams |
| Yeast Extract | 3 grams |
| Peptone | 3 grams |
| Dextrose | 10 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 ML |
Sabouraud Media
| Chemical | Usage Amount |
|---|---|
| Cycloheximide (Optional) | 10 mg |
| Chloramphenicol (Optional) | 0.5 grams |
| Peptone | 5 grams |
| Dextrose | 20 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 ML |
Freezing Media
| Chemical | Usage Amount |
|---|---|
| Glycerin | 50 grams |
| Ascorbic Acid | 15 grams |
| Liquid YPD/MYPG | Fill to 100 ML |
Beef Broth Media
| Chemical | Usage Amount |
|---|---|
| Beef Broth(No preservatives) | 500 mL |
| NaCI (can substitute non-iodized or sea salt) | 50-200 grams |
| Peptone | 5 grams |
| Dextrose | 10 grams |
| Agar(optional) | 17 grams |
| Distilled Water | Fill to 1000 ML |
Wild Yeast Screening Media
| Chemical | Usage Amount |
|---|---|
| Peptone | 5 grams |
| Yeast Extract | 3 grams |
| Malt Extract | 3 grams |
| Dextrose | 5 grams |
| CuSO4 | 310 mg |
| Distilled Water | Fill to 1000 ML |
LCYM/LCSM Media (for wild yeast and diastaticus) [7]
| Chemical | Usage Amount |
|---|---|
| Ammonium chloride | 0.5 grams |
| Dipotassium ortho phosphate | 1.1 grams |
| Cupric sulphate (anhydrous) | 0.55 grams |
| Dextrose | 10 grams |
| DME | 2 grams |
| Peptone | 2 grams |
| Yeast Extract | 4 grams |
| Agar | 20 grams |
| Distilled/De-ionized Water | Fill to 1000 mL |
Omega-optimized LCYM Media (Autoclaved for no longer than 15 minutes to preserve copper-containing ingredients. This media is specifically for detecting diastaticus; reported more reliable detection than regular LCSM and Weber diastaticus media; see this MTF thread by Laura Burns from Omega Yeast Labs and the associated write up)
| Chemical | Usage Amount |
|---|---|
| Ammonium sulfate | 0.5 grams |
| Dipotassium ortho phosphate | 1 gram |
| Cupric sulphate (anhydrous) | 0.6 grams |
| Dextrose | 10 grams |
| DME | 2 grams |
| Peptone | 2 grams |
| Yeast Extract | 4 grams |
| Agar | 20 grams |
| Distilled/De-ionized Water | Fill to 1000 mL |
Brettanomyces
A few different media can be used to isolate Brettanomyces but DBDM medium is commonly used. WLD with additions of cycloheximide can also be used. A simple Malt agar with cycloheximide has been shown to grow Brettanomyces that has been adapted to the brewing environment more efficiently than Dekkera medium (recommended by the European Brewing Convention, but not listed here) and universal beer medium (recommended by the Brewery Convention of Japan; also not listed here) [8]. DBDM may not allow all strains of Brettanomyces to grow [9].
Renouf et al. (2007) and Comitini et al. (2019) demonstrated that an "enrichment Brettanomyces bruxellensis" media called EBB is more efficient at first growing up Brettanomyces before trying to culture it on DBDM. Brettanomyces was allowed to grow for 80 days in the EBB media, and then streaked onto DBDM for selection for Brettanomyces (other wild yeast such as Hanseniaspora and Pichia grew much more readily than Brettanomyces that was cultured from wine grapes) [10][11].
See also: Wild Isolation of Brettanomyces.
EBB Media Recipe
| Chemical | Usage Amount |
|---|---|
| Red grape juice (possibly replaceable with DME?) | 200 mL/L |
| Ethanol | 40 mL/L |
| Malt extract | 1.5 g/L |
| Yeast extract | 1.5 g/L |
| (NH4)2 SO4 (sourced from Sigma-Aldrich) | 0.5 g/L |
| MgSO4 | 0.2 g/L |
| Tween 80 | 0.5 mL/L |
| Biphenyl (limits mold) | 0.2 g/L (w/v) |
| Chloramphenicol (limits bacteria) | 0.05 g/L (w/v) |
| Adjust pH to 5.0 with sodium hydroxide |
Notes: gentle agitation during growth (120 rpm).
DBDM Media Recipe
| Chemical | Usage Amount |
|---|---|
| Yeast nitrogen base (YNB) | 6.5 grams |
| Ethanol | 4% v/v |
| Cycloheximide | 10 mg |
| p-coumaric acid | 100 mg |
| Bromocresol Green | 22 mg |
| Agar | 20 grams |
| Distilled Water | Fill to 1000 mL |
WLD Media Recipe
| Chemical | Usage Amount |
|---|---|
| Cycloheximide | 4 grams |
| Yeast Extract | 4 grams |
| Pancreatic Digest of Casein(Peptone) | 5 grams |
| Dextrose | 50 grams |
| Monopotassium Phosphate | .55 grams |
| Potassium Chloride | 425 mg |
| Calcium Chloride | 125 mg |
| Magnesium Sulfate | 125 mg |
| Ferric Chloride | 2.5 mg |
| Manganese Sulfate | 2.5 mg |
| Bromocresol Green | 22 mg |
| Agar | 20 grams |
| Distilled Water | Fill to 1000 mL |
Malt Agar Media Recipe
Click here for directions on how to make this medium. The below recipe is using the Suzuki (2008) recipe instead of the recipe in the linked directions.
| Malt extract | 30 grams |
| Cycloheximide | 0.01 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 mL |
Yeast Growth Media
This is a simple non-synthetic yeast growth media shared by Cory Widmayer on Milk The Funk, and is based off of Leite et al. (2013). See Widmayer's post on MTF for directions on using this media (requires aeration). This media and method are claimed to grow Brettanomyces very quickly. Widmayer also provides a synthetic version of the recipe on the MTF post.
| Glucose or another sugar type | 100 grams |
| Yeast Extract | 90 grams |
| Distilled Water | Fill to 1000 mL |