Difference between revisions of "PH Meter"
(added MW102 storage solution link) |
(started a section for usage) |
||
| Line 56: | Line 56: | ||
"A simple test can be performed using Windex glass cleaner with ammonia and white distilled vinegar. The procedure is as follows: Turn your meter or tester on and place the probe in white distilled vinegar which is acidic (2.4ph) and the reading will be from 2.3 to 2.5 but must be below 3.0 then go to the Windex with ammonia which is alkaline. Your display should move very quickly up the scale to 10.5 – 11.2 but must go above 10.0. If the probe slowly moves up the scale then it is time to consider replacing it. If it does not go below 3.0 pH in vinegar and/or above the 10.0 pH in Windex then probe is dead." <ref>[http://www.milwaukeeinstruments.com/site/pdf/pH_Application_Info.pdf Milwaukee Instruments pH General Information Sheet. Retrieved 4/30/2015.]</ref> | "A simple test can be performed using Windex glass cleaner with ammonia and white distilled vinegar. The procedure is as follows: Turn your meter or tester on and place the probe in white distilled vinegar which is acidic (2.4ph) and the reading will be from 2.3 to 2.5 but must be below 3.0 then go to the Windex with ammonia which is alkaline. Your display should move very quickly up the scale to 10.5 – 11.2 but must go above 10.0. If the probe slowly moves up the scale then it is time to consider replacing it. If it does not go below 3.0 pH in vinegar and/or above the 10.0 pH in Windex then probe is dead." <ref>[http://www.milwaukeeinstruments.com/site/pdf/pH_Application_Info.pdf Milwaukee Instruments pH General Information Sheet. Retrieved 4/30/2015.]</ref> | ||
| + | ==Using a pH Meter== | ||
| + | (This section in progress) | ||
| + | |||
| + | * Carbonic acid can affect pH readings. If testing a beer that has CO2 in it, either residual from fermentation or from keg/bottle carbonation, let the beer warm to room temperature and swirl the beer to try and get all of the CO2 out of solution before taking a pH measumrement <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1153226544705558/?comment_id=1153275738033972&reply_comment_id=1153651057996440&total_comments=5&comment_tracking=%7B%22tn%22%3A%22R%22%7D Conversation with Gareth Young on MTF. 09/23/2015.]</ref>. | ||
| + | |||
==External Articles== | ==External Articles== | ||
* [http://www.homebrewtalk.com/showthread.php?t=302256 AJ Delange's Guide on How to Calibrate and Use pH Meters for Homebrewers]. | * [http://www.homebrewtalk.com/showthread.php?t=302256 AJ Delange's Guide on How to Calibrate and Use pH Meters for Homebrewers]. | ||
Revision as of 10:58, 24 September 2015
A pH Meter is not only considered by some to be an essential tool for all grain homebrewing and commercial brewing, but is also a very useful tool to have for making sour beer. For examples of when an accurate pH reading is useful in the sour brewing process, see the Mixed Fermentation, Sour Worting, and Lactobacillus pages.
Contents
Recommend pH Meters
High End pH Meters
Higher end pH meters recommended by our members and others.
- Mi150 pH and temperature Laboratory Bench Meter (runs about $250 US) [1]
- UltraBasic Benchtop Meters (discontinued, but may find used at a good price) [2].
Budget pH Meters
pH meters can range from $30 to $500+. The problem with many of the cheaper pH meters is that their reliability and longevity are often questionable [3]. Commonly, two pH meters are recommended by trustworthy sources (AJ Delange and Kai Troester).
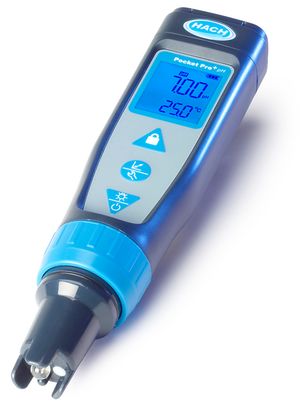
Hach Pocket Pro+ pH Tester with Replaceable Sensor.
Features:
- This pH meter is recommended by AJ Delange [3][4].
- Does not require storage solution (there is an o-ring seal on the cap, which only needs to have a few drops of distilled water in it).
- Replaceable sensor.
- Stability [3].
- 2 Buffer calibration.
- Electrode longevity [3].
- Junction resistant to fouling by sugars, proteins [3].
- Lets the user decide when to accept a buffer calibration reading [3].
- ATC [3].
- Automatic buffer recognition [3].
Disadvantages:
- Despite having an automatic calibration message, this pH meter still often needs to be calibrated before each brew day [3].
- The buttons have been reported to be touchy by some.
- Poor instructions that come with the unit. Use the website manual instead of the instructions that come with it.
Milwaukee MW102 pH/Temp Meter (updated/upgraded from SM101 and MW101).
Features:
- This pH meter is recommended by Kai Troester (SM101 originally) [5].
- 2 Buffer calibration (calibration video)
- ATC.
- Stability [6].
- Separate pH and temperature probes (can use as RTD for temp monitoring) [7].
- Storage solution container screws into probe [7].
- 0.02+/- accuracy.
- Automatic temperature compensation.
- Product specs comparison chart.
- Milwaukee Instruments pH General Information Sheet
Disadvantages:
- This pH meter usually needs to be calibrated before each brew day.
- Probe replacements appear to be difficult to find. Here is one vendor. eBay has been reported to be another option [8]. Here is a seller that PJ Dunn has had good results from, although he reported slow shipping.
- Probe has to be stored in Milwaukee MA9015 Storage Solution.
Buffer Solution
Buffer solution will be required to calibrate a pH meter. In general, it is recommended that a pH meter have a 2 point calibration method, which requires 4.0 buffer solution and 7.0 buffer solution. Many buffer solutions for pH meter calibration can be found on Amazon or other retailers. Note that these buffer solutions do have expiration dates, and using expired calibration solutions can throw off pH meter readings.
Testing the Probe
The probes on pH meters generally have a limited life, depending on the quality of the unit. Milwakuee Instruments describes the following method for testing the life of the probe:
"A simple test can be performed using Windex glass cleaner with ammonia and white distilled vinegar. The procedure is as follows: Turn your meter or tester on and place the probe in white distilled vinegar which is acidic (2.4ph) and the reading will be from 2.3 to 2.5 but must be below 3.0 then go to the Windex with ammonia which is alkaline. Your display should move very quickly up the scale to 10.5 – 11.2 but must go above 10.0. If the probe slowly moves up the scale then it is time to consider replacing it. If it does not go below 3.0 pH in vinegar and/or above the 10.0 pH in Windex then probe is dead." [9]
Using a pH Meter
(This section in progress)
- Carbonic acid can affect pH readings. If testing a beer that has CO2 in it, either residual from fermentation or from keg/bottle carbonation, let the beer warm to room temperature and swirl the beer to try and get all of the CO2 out of solution before taking a pH measumrement [10].
External Articles
- AJ Delange's Guide on How to Calibrate and Use pH Meters for Homebrewers.
- Kai Troester's Guide on Purchasing a pH Meter for Homebrewers.
- Measuring Mash pH in Practice, Accidentalis Blog.
- Bru'n Water on how to use a pH meter.
- pH Readings of Commercial Beers, Embrace the Funk Blog, Brandon Jones.
- How to Care for Your pH Meter; BitesizeBio.
References
- ↑ Conversation with Jonathan Newman from The Virginia Beer Company on Milk The Funk. 04/19/2015.
- ↑ Conversation with Brandon Jones of Embrace The Funk on Milk The Funk. 04/21/2015.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 AJ Delange. Homebrewtalk Thread. Posted 02/08/2012. Retrieved 04/18/2015.
- ↑ Testing the Hach Pocket Pro+ by AJ Delange Homebrewtalk. Retrieved 04/18/2015.
- ↑ Kai Troester. Braukeiser. Updated 09/23/2009. Retrieved 04/18/2015.
- ↑ Comment by AJ Delange on Homebrewtalk. Posted 08/01/2014. Retrieved 04/18/2015.
- ↑ 7.0 7.1 Conversation with Dustin Carver on Milk THe Funk. 4/18/2015.
- ↑ Conversation with PJ Dunn on MTF. 05/03/2015.
- ↑ Milwaukee Instruments pH General Information Sheet. Retrieved 4/30/2015.
- ↑ Conversation with Gareth Young on MTF. 09/23/2015.