Difference between revisions of "Pediococcus"
| Line 61: | Line 61: | ||
''P. damnosus'', as well as many other bacteria (but not all bacteria), have been shown to alter the genes of most, but not all, [[Saccharomyces]] species (and perhaps [[Brettanomyces]]), including S. cerevisiae, in a way that changes how they ferment sugars, and essentially forms a symbiotic environment with the yeast. Normally, ''Saccharomyces'' will always ferment glucose and only glucose when glucose is present. It will ignore other sugars until the glucose is gone. Biologically speaking, when the presence of glucose in the yeast's environment shuts down the yeast's ability to ferment any other sugar besides glucose, this is called "glucose repression" <ref name="cross-kingdom">[http://weitzlab.seas.harvard.edu/files/weitzlab/files/2014_cell_jarosz.pdf Cross-Kingdom Chemical Communication Drives a Heritable, Mutually Beneficial Prion-Based Transformation of Metabolism. 2014. Daniel F. Jarosz, Jessica C.S. Brown, Gordon A. Walker, Manoshi S. Datta, W. Lloyd Ung, Alex K. Lancaster, Assaf Rotem, Amelia Chang, Gregory A. Newby,David A. Weitz, Linda F. Bisson, and Susan Lindquist. Cell. 2014 Aug 28;158(5):1083-93.]</ref>. ''Saccharomyces'' and ''Brettanomyces bruxellensis'' (it is currently not known if other ''Brett'' species other than ''B. bruxellensis'' have this ability) have a gene called "GAF+" that when expressed actually allows it to bypass this "glucose repression" and ferment the other sugars simultaneously, but it is generally not expressed except by a very small number of cells <ref>[http://www.cell.com/cell/abstract/S0092-8674(14)00974-X An Evolutionarily Conserved Prion-like Element Converts Wild Fungi from Metabolic Specialists to Generalists. Daniel F. Jarosz, Alex K. Lancaster, Jessica C.S. Brown, Susan Lindquist. Cell. Volume 158, Issue 5, p1072–1082, 28 August 2014]</ref>. | ''P. damnosus'', as well as many other bacteria (but not all bacteria), have been shown to alter the genes of most, but not all, [[Saccharomyces]] species (and perhaps [[Brettanomyces]]), including S. cerevisiae, in a way that changes how they ferment sugars, and essentially forms a symbiotic environment with the yeast. Normally, ''Saccharomyces'' will always ferment glucose and only glucose when glucose is present. It will ignore other sugars until the glucose is gone. Biologically speaking, when the presence of glucose in the yeast's environment shuts down the yeast's ability to ferment any other sugar besides glucose, this is called "glucose repression" <ref name="cross-kingdom">[http://weitzlab.seas.harvard.edu/files/weitzlab/files/2014_cell_jarosz.pdf Cross-Kingdom Chemical Communication Drives a Heritable, Mutually Beneficial Prion-Based Transformation of Metabolism. 2014. Daniel F. Jarosz, Jessica C.S. Brown, Gordon A. Walker, Manoshi S. Datta, W. Lloyd Ung, Alex K. Lancaster, Assaf Rotem, Amelia Chang, Gregory A. Newby,David A. Weitz, Linda F. Bisson, and Susan Lindquist. Cell. 2014 Aug 28;158(5):1083-93.]</ref>. ''Saccharomyces'' and ''Brettanomyces bruxellensis'' (it is currently not known if other ''Brett'' species other than ''B. bruxellensis'' have this ability) have a gene called "GAF+" that when expressed actually allows it to bypass this "glucose repression" and ferment the other sugars simultaneously, but it is generally not expressed except by a very small number of cells <ref>[http://www.cell.com/cell/abstract/S0092-8674(14)00974-X An Evolutionarily Conserved Prion-like Element Converts Wild Fungi from Metabolic Specialists to Generalists. Daniel F. Jarosz, Alex K. Lancaster, Jessica C.S. Brown, Susan Lindquist. Cell. Volume 158, Issue 5, p1072–1082, 28 August 2014]</ref>. | ||
| − | When ''P. damnosus'' lives together with ''Saccharomyces'', a chemical is produced by ''P. damnosus'' that essentially "turns on" the GAF+ gene in ''Saccharomyces''. Not only does ''Sacch'' then have the ability to ferment other sugars at the same time as glucose, but it produces less alcohol. Viability over time is also increased in ''Sacch'' cells that express this gene versus those that don't. In wild fermentation of grapes, the GAF+ ''Sacch'' strains thrived over the other types of fungi that were found on the wild grapes | + | When ''P. damnosus'' lives together with ''Saccharomyces'', a chemical is produced by ''P. damnosus'' that essentially "turns on" the GAF+ gene in ''Saccharomyces''. Not only does ''Sacch'' then have the ability to ferment other sugars at the same time as glucose, but it produces less alcohol. Viability over time is also increased in ''Sacch'' cells that express this gene versus those that don't. In wild fermentation of grapes, the wild GAF+ ''Sacch'' strains thrived over the other types of fungi that were found on the wild grapes. This led the researchers of the referenced study to speculate that the GAF+ gene may play a role in preventing other fungi from thriving. It is theorized that this creates benefits for both microorganisms, which are often found together in the wild during fermentation of fruit; the bacteria isn't killed by higher alcohol levels, and the yeast has a broader food source. Furthermore, once a ''Saccharomyces'' cell expresses the gene, it will continue to pass this gene onto it's offspring. In winemaking, this is the cause of arrested wine fermentations due to the lower amount of alcohol produced (in the referenced study, the GAF- ''Sacch'' cells fermented out a must to 12% ABV wine, and the GAF+ ''Sacch'' cells fermented out an 8% ABV wine using the same must) <ref name="cross-kingdom"></ref>. However, the implications of this in sour beer brewing are much different and have yet to be further explored. |
Of all the ''Lactobacillus'' species that have been studied for this behavior (''L. brevis'', ''L. hilgardii'', ''L. plantarum'', and ''L. kunkeei''), only ''L. kunkeei'' was shown to induce the GAF+ gene. Different species of bacteria of ''Staphylococcus'', ''Micrococcus'', ''Bacillus'', ''Listeria'', ''Paenibacillus'', ''Gluconobacter'', ''Sinorhizobium'', ''Escherichia'', ''Serriatia'', and all ''Pediococcus'' species tested also influenced the GAF+ gene in ''Saccharomyces'' <ref name="cross-kingdom"></ref>. | Of all the ''Lactobacillus'' species that have been studied for this behavior (''L. brevis'', ''L. hilgardii'', ''L. plantarum'', and ''L. kunkeei''), only ''L. kunkeei'' was shown to induce the GAF+ gene. Different species of bacteria of ''Staphylococcus'', ''Micrococcus'', ''Bacillus'', ''Listeria'', ''Paenibacillus'', ''Gluconobacter'', ''Sinorhizobium'', ''Escherichia'', ''Serriatia'', and all ''Pediococcus'' species tested also influenced the GAF+ gene in ''Saccharomyces'' <ref name="cross-kingdom"></ref>. | ||
Revision as of 13:22, 2 August 2015
Pediococcus (often referred to as Pedio) are Gram-positive lactic acid bacteria (LAB) used in the production of Belgian style beers where additional acidity is desirable. They are native to plant material and fruits [1], and often found in spontaneously fermented beer as the primary source of lactic acid production (with P. damnosus being the only species identified in such beers) [2][3]. They are facultative anaerobes, meaning that they are capable of metabolism in both aerobic and anaerobic environments. Strains found in beer are hop tolerant [4]. Due to their continued metabolism of longer chain polysaccharides, acid production will increase with storage time. Pedio can form a pellicle.
Pediococcus may also cause “ropiness” (also called a "sick beer") due to the production of exopolysaccharides. "Ropy" or "sick" beer is more viscous and, in extreme circumstances, can form strands. Sickness effects mostly the mouthfeel and appearance of beer, and may have no influence on the flavor. Pediococcus species can also produce diacetyl with extended storage time. Brettanomyces can break down exopolysaccharides and diacetyl produced by Pediococcus and the two are often used together.
See Lactobacillus, Brettanomyces, Saccharomyces, and Mixed Cultures charts for other commercially available cultures.
Commercial Pediococcus Cultures
| Name | Mfg# | Taxonomy | Note |
|---|---|---|---|
| White Labs | WLP661 | damnosus | High diacetyl producer and slow growing. Fermentation temp: 70-85°F (21-29.4° C). Attenuation: 65% |
| Wyeast | 5733 | damnosus | May cause “ropiness” and produce low levels of diacetyl with extended storage time. Temp range: 60-95° F (15-35° C). |
| RVA Yeast Labs | RVA 601 | damnosus | Lactic acid bacteria used in souring Belgian-style beers such as gueze and Lambic. Acid production increases with storage. Temperature range is 60-95º F. |
Manufacturer Tips
Wyeast on 5733
"If using 1 pack of 5733 per 5 gallon batch; and either adding to secondary after alcoholic fermentation is complete, or co-inoculating with a Sacch' strain, then a starter would not be necessary. If you did want or need to propagate, I'd recommend 2 liters of 1.030-1.040 wort per pack, incubated at 80-90*F, without agitation." - Michael Dawson, Wyeast.
"For propagation, we recommend using 1.040 OG wort and incubating at 30-35*C without aeration for 48-96 hours; pH drop will indicate when it's ready to pitch. For co-inoculation or post-primary addition, we recommend 0.5 million cells per mL, which is the equivalent of 1 pack in 5 gallons/20 liters. For larger volumes, you can propagate and inoculate with the starter culture at a rate 2.5-5% of the total volume of the main batch." - Michael Dawson, Wyeast.
White Labs on WLP661
"That one does well in 70-85 deg F. You can do a starter, but you shouldn't have to if you are doing a 5 gallon batch. It does take a while to sour, so just be patient and let it do it's thing." Sarah Neel, Sales and Customer Service, White Labs
Tips From MTF
The Rare Barrel
"We just performed an interesting experiment at The Rare Barrel with pedio. Jay, our head brewer and blender, wanted a more acidic beer that we could use as a blending component while also growing up our diminishing pedio culture. So we racked 2 oak barrels of gold fermented with Brettanomyces claussenii and White Labs Pediococcus damnosus (WLP661) into one of our 30 bbl batches of 12*p gold wort that had been acidified to about 4.5 pH in the kettle using lactic acid (our first hot side experiment!). No oxygen, really wanted to encourage the bacteria. Within 10 days the pH was 3.6 and the gravity 10.7. We were all surprised how quickly the pedio was working. We eventually racked a "splash" of fermenting gold with BSI Brett D and BSI Lacto D to drop the gravity. The beer had a bright acidity quickly and I was surprised at how well rounded the flavors were when we racked into barrels after a month in the fermenting vessel.
This might be a little harder to do at home, but I think there's potential for interesting results. Pediococcus shines long term traditionally so I agree with the posts above, if you're going for quick acidity I'd go lacto, but I plan on playing around with early Pediococcus fermentations at home." - Mike Makris from The Rare Barrel [5].
Metabolism
Lactic Acid Production
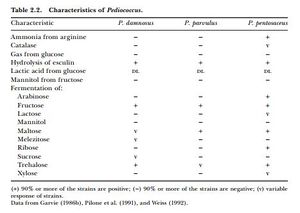
About 90% of sugar metabolized by Pediococcus produces lactic acid. It does so by homolactic fermentation (same EMP pathway as Lactobacillus homolactic fermentation), although some species/strains can convert glycerol to lactic acid, acetic acid, acetoin, and CO2 under aerobic conditions (P. damnosus is not in this category) [7]. P. damnosus can ferment glucose, sucrose, and galactose. Some strains of P. damnosus can ferment maltose and sucrose [1].
Growth and Environment
P. damnosus is sensitive to temperature and pH. It is unable to grow at a pH of 8 or higher or at 35°C. The optimal growth occurs at 22°C and 5.5 pH. P. damnosus is sensitive to environments that contain NaCl, and will not grow with concentrations of 4% NaCl [1].
One study showed that optimal growth was observed in MRS media with an initial pH of 6.7, and allowed to ferment down to a pH of 4.14 naturally from fermentation. The addition of bacteriological peptone, MnSO4, and Tween 80 also increased activity [8].
"Ropy" or "Sick" Beer
Some strains of P. damnosus can cause a beer (or wine) to go "ropy". This "ropiness" is actually caused by production of exopolysaccharides (EPS) in the form of β-glucans (beta glucans). A small amount of β-glucan is adequate enough to affect the visible viscosity of beer or wine. The gene known as Dps has been identified with the production of β-glucan/EPS. Pediococci that are ropy have been found to be more acid, alcohol, and SO2 tolerant than other Pediococci. The thickness of the ropiness is increased with the presence of malic acid [9].
One study showed that the production of β-glucan coincided with the end of the growth phase of Pediococcus. While small amounts of β-glucan were produced during growth, after 2 days of growth, β-glucan production increased as growth slowed. β-glucan production stopped when growth stopped. This study showed that β-glucan production is linked to Pediococcous growth, producing more towards the end of growth. This would explain why beer containing Pedio often goes ropy shortly after naturally carbonating in the bottle. This study found that other variables were not factors in the production of β-glucan, such differing levels of alcohol (although alcohol interacts with the β-glucan in a way that makes the viscosity seem thicker). The study also found that the lack of agitation increased the β-glucan production (wine makers will often agitate or aerate ropy wine to cure the wine from ropiness). A higher initial pH encourages higher growth (5.5+), which increases β-glucan production. A lower initial pH (3.5), decreases growth and β-glucan production. A higher concentration of glucose increased growth and β-glucan production. A low starting pH decreases growth, and therefore decreases β-glucan production. Glocuse is needed for β-glucan production. While fructose alone is mostly insufficient, a combination of glucose and fructose was slightly more efficient than glucose alone [9].
It has been observed that Lactobacillus species can produce EPS (Lactococcus lactis, Lactobacillus delbrueckii, Lactobacillus casei, and Lactobacillus helveticus) [9].
Other Metabolites
P. damnosus can produce high amounts of diacetyl during lactic acid production [10][11]. P. damnosus also produces an antimicrobial compound called pediocin PD-1, which can inhibit several bacteria spp including O. oeni [12][13].
Mixed Culture Influence
- Editor's note: special thanks to Richard Preiss of Escarpment Yeast Laboratories for helping to interpret the science referenced this section.
P. damnosus, as well as many other bacteria (but not all bacteria), have been shown to alter the genes of most, but not all, Saccharomyces species (and perhaps Brettanomyces), including S. cerevisiae, in a way that changes how they ferment sugars, and essentially forms a symbiotic environment with the yeast. Normally, Saccharomyces will always ferment glucose and only glucose when glucose is present. It will ignore other sugars until the glucose is gone. Biologically speaking, when the presence of glucose in the yeast's environment shuts down the yeast's ability to ferment any other sugar besides glucose, this is called "glucose repression" [14]. Saccharomyces and Brettanomyces bruxellensis (it is currently not known if other Brett species other than B. bruxellensis have this ability) have a gene called "GAF+" that when expressed actually allows it to bypass this "glucose repression" and ferment the other sugars simultaneously, but it is generally not expressed except by a very small number of cells [15].
When P. damnosus lives together with Saccharomyces, a chemical is produced by P. damnosus that essentially "turns on" the GAF+ gene in Saccharomyces. Not only does Sacch then have the ability to ferment other sugars at the same time as glucose, but it produces less alcohol. Viability over time is also increased in Sacch cells that express this gene versus those that don't. In wild fermentation of grapes, the wild GAF+ Sacch strains thrived over the other types of fungi that were found on the wild grapes. This led the researchers of the referenced study to speculate that the GAF+ gene may play a role in preventing other fungi from thriving. It is theorized that this creates benefits for both microorganisms, which are often found together in the wild during fermentation of fruit; the bacteria isn't killed by higher alcohol levels, and the yeast has a broader food source. Furthermore, once a Saccharomyces cell expresses the gene, it will continue to pass this gene onto it's offspring. In winemaking, this is the cause of arrested wine fermentations due to the lower amount of alcohol produced (in the referenced study, the GAF- Sacch cells fermented out a must to 12% ABV wine, and the GAF+ Sacch cells fermented out an 8% ABV wine using the same must) [14]. However, the implications of this in sour beer brewing are much different and have yet to be further explored.
Of all the Lactobacillus species that have been studied for this behavior (L. brevis, L. hilgardii, L. plantarum, and L. kunkeei), only L. kunkeei was shown to induce the GAF+ gene. Different species of bacteria of Staphylococcus, Micrococcus, Bacillus, Listeria, Paenibacillus, Gluconobacter, Sinorhizobium, Escherichia, Serriatia, and all Pediococcus species tested also influenced the GAF+ gene in Saccharomyces [14].
See Also
Additional Articles on MTF Wiki
- Alternative Bacteria Sources
- Spontaneous Fermentation
- Mixed Fermentation
- Mixed Cultures
- Scientific Publications
- Lactobacillus
External Resources
- UC Davis article stub on Pediococcus damnosus.
- The Genera of Lactic Acid Bacteria, by W.H.N Holzapfel, Brian J.B. Wood. Partially free.
References
- ↑ 1.0 1.1 1.2 Viticulture & Enology. UC Davis website. Pedioccous damnosus. Retreived 07/28/2015.
- ↑ The Microbial Diversity of Traditional Spontaneously Fermented Lambic Beer. Freek Spitaels, Anneleen D. Wieme, Maarten Janssens, Maarten Aerts, Heide-Marie Daniel, Anita Van Landschoot, Luc De Vuyst, Peter Vandamme. April 18, 2014.
- ↑ Scientific_Publications#Lambic_and_Spontaneous_Fermentation Multiple Scientific publications linked on MTF.
- ↑ Comparative genome analysis of Pediococcus damnosus LMG 28219, a strain well-adapted to the beer environment. Isabel Snauwaert, Pieter Stragier, Luc De Vuyst and Peter Vandamme. April 2015.
- ↑ Conversation with Mike Makris on Milk The Funk.
- ↑ Wine Microbiology. Practical Applications and Procedures. Kenneth C. Fugelsang, Charles G. Edwards.
- ↑ Encyclopedia of Food Microbiology. Pediococcus. Carl A. Batt. Academic Press, Sep 28, 1999 .
- ↑ Nel HA, Bauer R, Vandamme EJ, Dicks LM. Growth optimization of Pediococcus damnosus NCFB 1832 and the influence of pH and nutrients on the production of pediocin PD-1. Department of Microbiology, University of Stellenbosch, Stellenbosch, South Africa. Dec 2001.
- ↑ 9.0 9.1 9.2 9.3 Glucose fermentation kinetics and exopolysaccharide production by ropy Pediococcus damnosus IOEB8801. Emilie Walling, Marguerite Dols-Lafargue, Aline Lonvaud-Funel. Food Microbiology Volume 22, Issue 1, January 2005, Pages 71–78.
- ↑ Identification of pediococci by ribotyping. R. Satokari, T. Mattila-Sandholm and M.L. Suihko. Journal of Applied Microbiology 2000, 88, 260–265.
- ↑ The Microbiology of Malting and Brewing. Nicholas A. Bokulicha, and Charles W. Bamforth. June 2013.
- ↑ Growth optimization of Pediococcus damnosus NCFB 1832 and the influence of pH and nutrients on the production of pediocin PD-1. H.A. Nel1, R. Bauer, E.J. Vandamme and L.M.T. Dicks. Jan 2002.
- ↑ Purification, partial amino acid sequence and mode of action of pediocin PD-1, a bacteriocin produced by Pediococcus damnosus NCFB 1832. Bauer R, Chikindas ML, Dicks LM. May 2005.
- ↑ 14.0 14.1 14.2 Cross-Kingdom Chemical Communication Drives a Heritable, Mutually Beneficial Prion-Based Transformation of Metabolism. 2014. Daniel F. Jarosz, Jessica C.S. Brown, Gordon A. Walker, Manoshi S. Datta, W. Lloyd Ung, Alex K. Lancaster, Assaf Rotem, Amelia Chang, Gregory A. Newby,David A. Weitz, Linda F. Bisson, and Susan Lindquist. Cell. 2014 Aug 28;158(5):1083-93.
- ↑ An Evolutionarily Conserved Prion-like Element Converts Wild Fungi from Metabolic Specialists to Generalists. Daniel F. Jarosz, Alex K. Lancaster, Jessica C.S. Brown, Susan Lindquist. Cell. Volume 158, Issue 5, p1072–1082, 28 August 2014