Difference between revisions of "Brettanomyces Propagation Experiment"
m |
m |
||
| Line 7: | Line 7: | ||
==Procedures== | ==Procedures== | ||
| − | A strain of ''Brettanomyces'' believed to be B. bruxellensis was isolated by Trent from a bottle of Orval in 2012. This ''Brettanomyces'' strain was subjected to four different propagation treatments: aerobic (stir plate set to high with a foil cover), semi-aerobic (orbital shaker set to 80 RPM's with a foil cover), anaerobic with constant agitation (Co2 purged and then set on an orbital shaker set to 80 RPM's with an airlock), and still (foil cover, no agitation). All treatments were duplicated. | + | A strain of ''Brettanomyces'' believed to be the species ''B. bruxellensis'' was isolated by Trent from a bottle of Orval in 2012. This ''Brettanomyces'' strain was subjected to four different propagation treatments: aerobic (stir plate set to high with a foil cover), semi-aerobic (orbital shaker set to 80 RPM's with a foil cover), anaerobic with constant agitation (Co2 purged and then set on an orbital shaker set to 80 RPM's with an airlock), and still (foil cover, no agitation). All treatments were duplicated. |
The initial inoculum of ''Brettanomyces'' was grown in 3 steps to 300 mL slurry from a single colony. For each treatment 35 mL of the inoculum ''Brettanomyces'' was added to 230 mL of 10°P wort in 500 mL Erlenmeyer flasks for an initial cell density of 112.87 million cells per mL. | The initial inoculum of ''Brettanomyces'' was grown in 3 steps to 300 mL slurry from a single colony. For each treatment 35 mL of the inoculum ''Brettanomyces'' was added to 230 mL of 10°P wort in 500 mL Erlenmeyer flasks for an initial cell density of 112.87 million cells per mL. | ||
Revision as of 16:20, 4 September 2019
This page documents an experiment conducted by Mark Trent to compare different aeration conditions for Brettanomyces during propagation. It was originally posted by Trent on the Milk The Funk Facebook Group. Significant and relevant follow up comments from other MTF members have been included in this write up.
Contents
Purpose
For brewers there have generally been two approaches to making starters to propagate Brettanomyces. Basically, the first approach is to create a semi-aerobic environment. The second approach is to use a stir plate. Using a stir plate will introduce more oxygen, which can create a a more acetic starter. Usually the starter beer is decanted so that the acetic acid is not added to the beer. See the Brett Starter Information page for details.
It is thought that oxygen will encourage growth for Brettanomyces. To test this hypothesis, Mark Trent executed the following controlled experiment. This experiment aims to determine how aeration and agitation affects Brett' cell growth and pH of the starter wort.
Procedures
A strain of Brettanomyces believed to be the species B. bruxellensis was isolated by Trent from a bottle of Orval in 2012. This Brettanomyces strain was subjected to four different propagation treatments: aerobic (stir plate set to high with a foil cover), semi-aerobic (orbital shaker set to 80 RPM's with a foil cover), anaerobic with constant agitation (Co2 purged and then set on an orbital shaker set to 80 RPM's with an airlock), and still (foil cover, no agitation). All treatments were duplicated.
The initial inoculum of Brettanomyces was grown in 3 steps to 300 mL slurry from a single colony. For each treatment 35 mL of the inoculum Brettanomyces was added to 230 mL of 10°P wort in 500 mL Erlenmeyer flasks for an initial cell density of 112.87 million cells per mL.
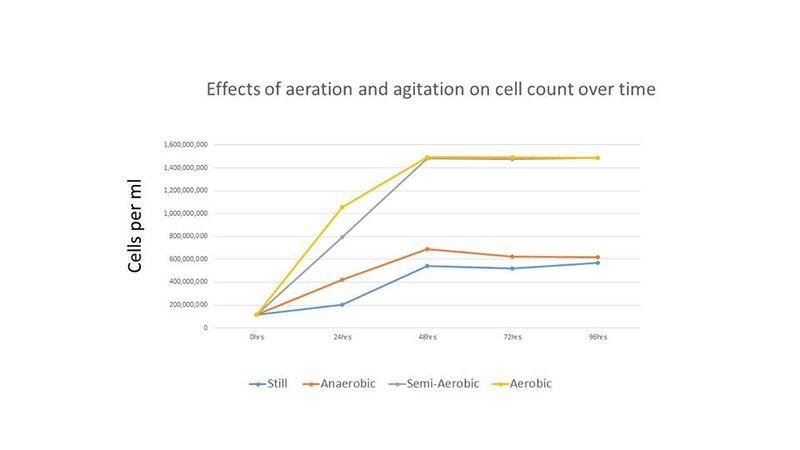
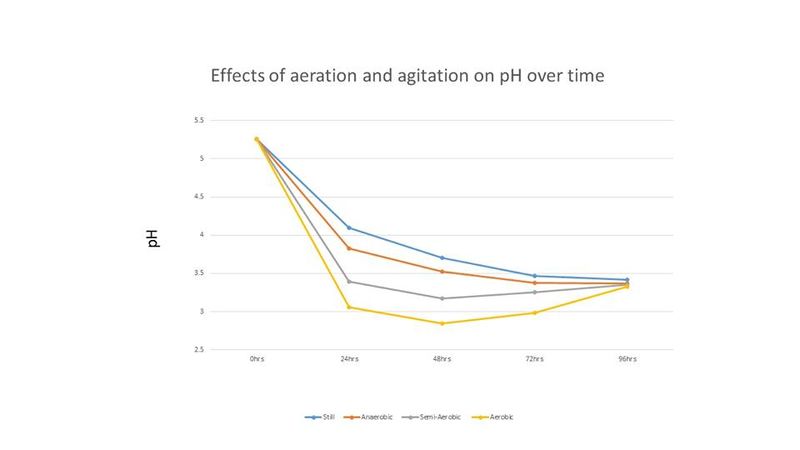
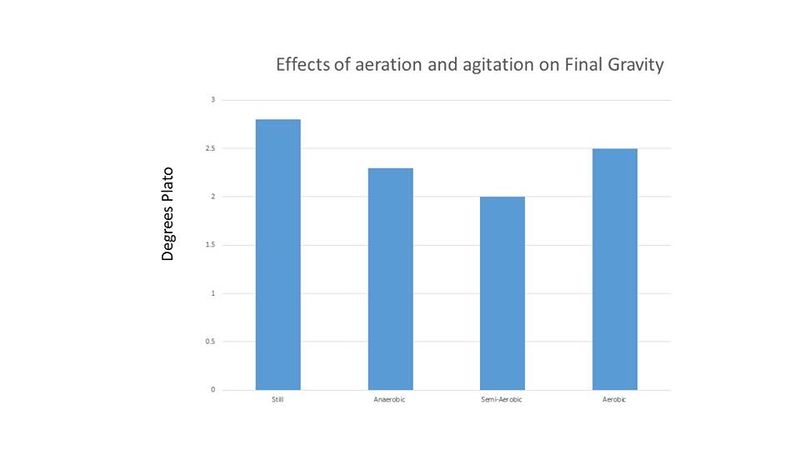
Cell counts and pH readings were taken every 24 hours until all treatments showed no or little increase in cell count. Cell counts were done with a hemocytometer. Final gravity for each treatment was recorded at the end of the experiment. The results of this experiment are shown below in figures 1-3.
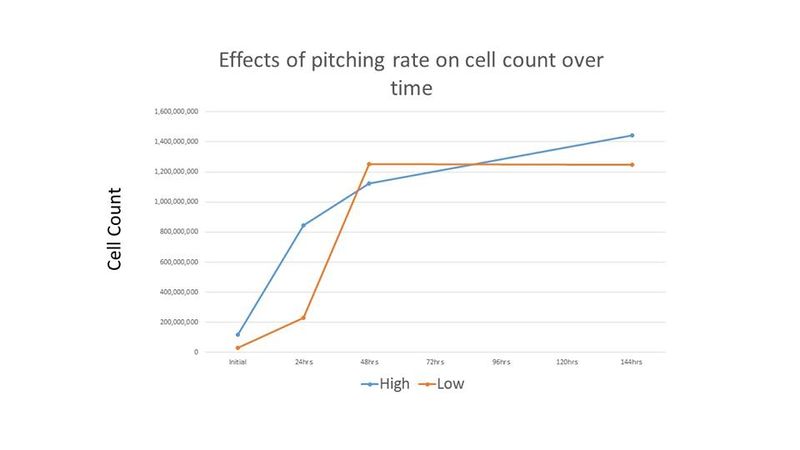
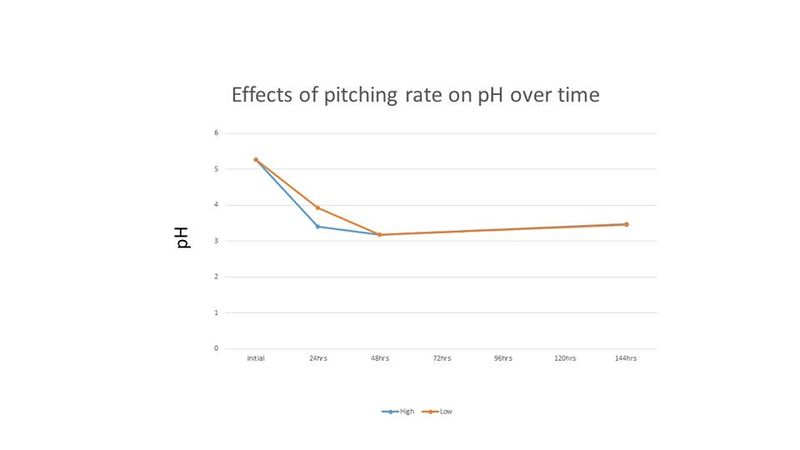
Because the cell counts were so high and the time to completion was so short, it was thought that these results may be due to the high level of initial cell count. Therefore, another experiment was performed comparing an initial cell count of 30 million cells per mL (low) and 120 million cells per mL (high). The treatments were duplicated. The Brettanomyces inoculum was prepared as described above and each treatment was prepared in 265 mL of wort and incubated on an orbital shaker at 80 RPM and 26°C. Because "life got in the way", cell counts and pH readings were only taken for the first 2 days, and then again on the 6th day. Both treatments in this second experiment resulted in a final gravity of 2°P on day 6. Figures 4-5 show the results of this experiment.
Results
Discussion
The results indicate that for this strain of Brettanomyces a near equal cell density can be achieved with aerated or semi-aerated propagation while anaerobic incubation under agitation or still incubation resulted in less than half the cell density when compared to the former treatments. While the pH decreased with more aeration during propagation, it is interesting that the pH of all treatments were near equal by the end of the experiment. The cell counts reached by the aerobic and semi-aerobic treatments were much higher than reported in the literature and the time to reach maximum cell density is much shorter than previous reports for propagation of Brettanomyces.
In Figure 2, the pH of the starter wort changed throughout the incubation period, with the aerobic and semi-aerobic treatments creating a lower pH from 24 hours to 72 hours. However, after 90 hours the pH for all treatments stabilized around the same pH (~3.4). Trent noted that although the pH of the final starters were nearly the same, the aerated condition tasted more acidic than the other three conditions [1].
This experiment demonstrated a significantly faster growth rate than many other growth rate studies [2]. It has been suggested by MTF member and microbiologist Richard Preiss that propagation times vary widely among Brettanomyces strains, and that the selected strain most likely exhibits faster reproduction than other strains [3]. Additionally, Trent's experiment did not demonstrate the same "second lag phase" as shown in Chad Yakobson's "The Brettanomyces Project" [2], including for the lower cell count treatment in the second experiment. Richard Preiss hypothesizes that one possibility to explain this is that the cells had a two step growth process applied in preparation for the experiment, and that this prior exposure to wort allows the Brettanomyces cells to adapt their metabolism to malt sugars. Preiss reports seeing something similar when growing Brettanomyces: when first grown in YPD, then in ~50 mL of wort, the culture then grows to maximum cell density within 24-72 hours (depending on strain). Preiss also notes that not all strains of Brettanomyces exhibit the "second lag phase", and that the chosen Brettanomyces strain may be one of these strains [4].
Oxygen levels between the four treatments were not measured with a Dissolved Oxygen meter. However, some observations were made by Trent that indicate a large difference in dissolved oxygen between the four treatments. The aerated (stir plate) treatment formed a significant vortex and the color (before growth) appeared milky due to the amount of agitation and perhaps gas dissolving into the media. Also a thick layer of foam was maintained on the aerated treatment throughout growth while only bubbles were observed on the semi-aerated [5].
Conclusion
This experiment supports prior starter recommendations (see Brettanomyces Starters) and indicates that oxygen exposure and agitation increase the cell growth rate of Brettanomyces, and that the amount of oxygen required for maximum growth is minimal, although more oxygen decreases the time to achieve maximum cell density. Also, higher levels of oxygen can possibly lead to more acetic acid production, although this was only measured sensorily and not with any kind of chemical analysis, and acetic acid was never identified as the cause of the acidic taste of the aerated treatment. This supports the results of several published studies that show that level of oxygen has a significant impact on level of acetic acid produced (see references: [6][7][8]). Agitation also appears to induce Brettanomyces growth as shown in Figure 1; the anaerobic treatment with agitation (80 RPM) showed slightly better growth than the treatment with a tin foil cover and no agitation (labelled "Still" in Figure 1).
Based on these findings and others, it is recommended that Brettanomyces is grown either on a stir plate with tin foil and decanted before pitching, or grown in a semi-aerobic condition. Semi-aerobic conditions can be met ideally by using an orbital shaker at 80 RPM. Those without an orbital shaker can use a stir plate set to the lowest setting, or an initial blast of pure oxygen followed by frequent manual agitation throughout the incubation time period.
It is recommended that this experiment is repeated with other strains of Brettanomyces. Lance Shaner of Omega Yeast Labs and Richard Preiss of Escarpment Yeast Labs volunteered to independently grow the strain of Brettanomyces used in the experiment to verify the maximum cell density of this strain (and thus the data collected - results pending).
See Also
Additional Articles on MTF Wiki
External Resources
References
- ↑ Conversation with Mark Trent regarding his Brett experiment and sensory analysis. 10/23/2015.
- ↑ 2.0 2.1 The Brettanomyces Project. Propagation and Batch Culture Results. Retrieved 11/05/2015.
- ↑ Comments by Richard Preiss on MTF regarding Mark Trent's Brett growth experiment. 10/23/2015.
- ↑ Comments by Richard Preiss on MTF regarding Mark Trent's Brett growth experiment. 10/23/2015.
- ↑ Comments by Mark Trent on MTF. 10/26/2015.
- ↑ Brettanomyces bruxellensis: effect of oxygen on growth and acetic acid production. Aguilar Uscanga, Délia1, and Strehaiano. 2003.
- ↑ Role of oxygen on acetic acid production by Brettanomyces/Dekkera in winemaking. Maurizio Ciani and Luisa Ferraro. April 1999.
- ↑ Acetic acid production by Dekkera/Brettanomyces yeasts. S.N. Feer. April 2002.