Difference between revisions of "Hops"
(added section for Kettle Hopping) |
m |
||
| Line 25: | Line 25: | ||
==Antimicrobial Properties== | ==Antimicrobial Properties== | ||
| − | Hops are known to have antimicrobial properties against gram positive bacteria. This includes bacteria which can be present in beer both as spoilage organisms and as intentionally added in sour and mixed fermentation beer such as ''[[Lactobacillus]]'' and ''[[Pediococcus]]''. Certain other bacteria found in beer such as ''Acetobacteraciae'' are gram negative and are not susceptible to the antimicrobial properties of hops. Certain Gram | + | Hops are known to have antimicrobial properties against gram positive bacteria. This includes bacteria which can be present in beer both as spoilage organisms and as intentionally added in sour and mixed fermentation beer such as ''[[Lactobacillus]]'' and ''[[Pediococcus]]''. Certain other bacteria found in beer such as ''Acetobacteraciae'' are gram negative and are not susceptible to the antimicrobial properties of hops. Certain Gram positive bacteria are known to be more resistant to the antimicrobial effects of hops. Multiple mechanisms have been proposed to explain why hops are antimicrobially active. |
One mechanism of the antimicrobial activity of hops is due to the role of alpha acids and similar hop acids (such beta acids and iso-α-acids) as ionophores, or compounds which can transport ions across cell membranes<ref name="Fernandez and Simpson, 1993"> [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.1993.tb02782.x/full Fernandez and Simpson (1993)] </ref> <ref name="Sakamoto and Konings, 2003"> [http://www.sciencedirect.com/science/article/pii/S0168160503001533 Sakamoto and Konings (2003)] </ref>. The protonated iso-α-acid (the form of the acid with an associated H+ ion, an H+ ion is a proton) is the antimicrobially active form. This means that for a beer with a given iso-α-acid concentration, the antimicrobial effects will be stronger at lower pH values because a greater percentage of the acid will be protonated. Protonated iso-α-acids act against bacteria by crossing into the cell and dissociating (releasing H+ ions from the iso-α-acid), therefore disrupting the cellular proton gradient which is necessary for cells to function, before binding an equal charge in metal ions and crossing back out of the cell. Cells with a resistance to hop bitter acids are better able to eject undissociated iso-α-acids from the cell and therefore preserve their proton gradients. The mechanism to expel iso-α-acids appears to be specific toward this type of compound rather than by a more general antimicrobial resistance mechanism such as multi-drug resistant bacteria possess<ref name="Sakamoto and Konings, 2003"/>. Hop resistant bacteria cultured in the absence of hop acids can lose their resistance if grown in an environment without antibacterial hop compounds<ref name="Fernandez and Simpson, 1993"/> and some hop resistant microbes need to be acclimated to hop acids by growth in sub-limiting levels of antibacterial acids before they are able to resist higher levels<ref name="Sakamoto and Konings, 2003"/>. | One mechanism of the antimicrobial activity of hops is due to the role of alpha acids and similar hop acids (such beta acids and iso-α-acids) as ionophores, or compounds which can transport ions across cell membranes<ref name="Fernandez and Simpson, 1993"> [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.1993.tb02782.x/full Fernandez and Simpson (1993)] </ref> <ref name="Sakamoto and Konings, 2003"> [http://www.sciencedirect.com/science/article/pii/S0168160503001533 Sakamoto and Konings (2003)] </ref>. The protonated iso-α-acid (the form of the acid with an associated H+ ion, an H+ ion is a proton) is the antimicrobially active form. This means that for a beer with a given iso-α-acid concentration, the antimicrobial effects will be stronger at lower pH values because a greater percentage of the acid will be protonated. Protonated iso-α-acids act against bacteria by crossing into the cell and dissociating (releasing H+ ions from the iso-α-acid), therefore disrupting the cellular proton gradient which is necessary for cells to function, before binding an equal charge in metal ions and crossing back out of the cell. Cells with a resistance to hop bitter acids are better able to eject undissociated iso-α-acids from the cell and therefore preserve their proton gradients. The mechanism to expel iso-α-acids appears to be specific toward this type of compound rather than by a more general antimicrobial resistance mechanism such as multi-drug resistant bacteria possess<ref name="Sakamoto and Konings, 2003"/>. Hop resistant bacteria cultured in the absence of hop acids can lose their resistance if grown in an environment without antibacterial hop compounds<ref name="Fernandez and Simpson, 1993"/> and some hop resistant microbes need to be acclimated to hop acids by growth in sub-limiting levels of antibacterial acids before they are able to resist higher levels<ref name="Sakamoto and Konings, 2003"/>. | ||
Revision as of 23:49, 12 December 2016
(In progress)
Hops are a dioecious (meaning that they have separate male and female plants) climbing bine whose female cones are used in brewing for flavor as well as for antimicrobial properties. Sour and funky brewers can use hops to help regulate lactic acid bacteria and control acid production to desired levels, especially in aged mixed-fermentation or spontaneous fermentation beers. Brewers who are interested in rapid acid production using quick/kettle souring techniques such as sour worting may wish to limit or avoid hop use before acidifying so that sufficient acid is produced on timescales of hours to a couple days.
Potential references (https://www.facebook.com/groups/MilkTheFunk/permalink/1190513557643523/?qa_ref=qd&comment_id=1195663997128479&comment_tracking=%7B%22tn%22%3A%22R%22%7D):
- http://onlinelibrary.wiley.com/doi/10.1002/j.2050-0416.1970.tb03259.x/abstract
- http://onlinelibrary.wiley.com/doi/10.1002/jib.40/full
- Kowaka, K., et al. "true value of aroma hops in brewing." Proceedings of the congress-European Brewery Convention. 1983.
- http://www.asbcnet.org/publications/journal/vol/abstracts/43-25.htm
- https://www.facebook.com/groups/MilkTheFunk/permalink/1228610483833830/
- https://www.facebook.com/groups/MilkTheFunk/permalink/1234538973240981/
Contents
Hop Composition
(in progress)
The main compounds of interest to brewers in hops are their bitter acids and oils. Alpha acids account for roughly 2-12% of dried hops by mass, beta acids account for roughly --- % and oils account for roughly 0.5-3%, though the exact percentages will vary depending on factors such as the hop varietal, growing region, harvest time, and growth conditions for the year.
The primary alpha acids (humulones) in hops are humulone, cohumulone, and adhumulone. The ratio of these individual acids to each other can vary much like total iso-α-acid percent, though generally the primary acids are -------. While alpha acids are insoluble in wort, the isomerized acids which are formed during boiling are soluble. Isomerization leads to roughly a 70%/30% split between cis and trans iso-α-acids respectively, with cis iso-α-acids being more stable over time and more bitter[1]. Alpha acids themselves do not taste bitter, but isomerized alpha acids (iso-α-acids) contribute to the bitterness of beer and have antimicrobial properties. Isocohumulone is often cited as being more harshly bitter than the other iso-α-acids, but studies of taste perception of individual iso-α-acids have not agreed with this. However Isocohumolone is slightly more soluble than the other acids and therefore a hop with a higher cohumulone composition may result in a beer with higher iso-α-acid for hops of equal iso-α-acid percent and use in brewing but different iso-α-acid breakdown[1]. Alpha acids are susceptible to oxidation and the alpha acid content of a hop will decrease with storage.
Beta Acids (lupulones) are similar in structure to alpha acids and have the analogous individual beta acids (lupulone, colupulone, adlupulone) to individual alpha acids. Beta acids are not able to isomerize and are therefore not soluble in wort unless they are chemically modified by a process such as oxidation. Oxidatized beta acids are soluble and can contribute to bitterness in beer. Oxidized beta acids are discussed more under aged hops.
There are three primary classes of oils in hops: hydrocarbons (~64% of the total oils), oxygenated compounds (~35% of the total oils), and sulfur compounds (≤1% of the total oils)[2]. Individual flavor and aroma active oils each have different thresholds, solubilities, and volatilities, and individual oils can have synergistic interactions with each other. The chemistry of hop oil taste perception is therefore very complicated and overall is not well understood. While sulfur compounds make up only a very small fraction of the total oils, they have a significant impact on hop flavor[2].
Antimicrobial Properties
Hops are known to have antimicrobial properties against gram positive bacteria. This includes bacteria which can be present in beer both as spoilage organisms and as intentionally added in sour and mixed fermentation beer such as Lactobacillus and Pediococcus. Certain other bacteria found in beer such as Acetobacteraciae are gram negative and are not susceptible to the antimicrobial properties of hops. Certain Gram positive bacteria are known to be more resistant to the antimicrobial effects of hops. Multiple mechanisms have been proposed to explain why hops are antimicrobially active.
One mechanism of the antimicrobial activity of hops is due to the role of alpha acids and similar hop acids (such beta acids and iso-α-acids) as ionophores, or compounds which can transport ions across cell membranes[3] [4]. The protonated iso-α-acid (the form of the acid with an associated H+ ion, an H+ ion is a proton) is the antimicrobially active form. This means that for a beer with a given iso-α-acid concentration, the antimicrobial effects will be stronger at lower pH values because a greater percentage of the acid will be protonated. Protonated iso-α-acids act against bacteria by crossing into the cell and dissociating (releasing H+ ions from the iso-α-acid), therefore disrupting the cellular proton gradient which is necessary for cells to function, before binding an equal charge in metal ions and crossing back out of the cell. Cells with a resistance to hop bitter acids are better able to eject undissociated iso-α-acids from the cell and therefore preserve their proton gradients. The mechanism to expel iso-α-acids appears to be specific toward this type of compound rather than by a more general antimicrobial resistance mechanism such as multi-drug resistant bacteria possess[4]. Hop resistant bacteria cultured in the absence of hop acids can lose their resistance if grown in an environment without antibacterial hop compounds[3] and some hop resistant microbes need to be acclimated to hop acids by growth in sub-limiting levels of antibacterial acids before they are able to resist higher levels[4].
(in progress) Another antimicrobial mechanism resulting from oxidative stress has been attributed to both iso-α-acids and humulinic acids[5]. Humulinic acids are either not bitter tasting or much less bitter than iso-α-acids but are similar in structure to and are formed from the degradation of iso-α-acids. This oxidative stress-driven antimicrobial activity is due to potential for oxidation-reduction (redox) reactions within bacterial cells between Mn2+ ions and these specific hop acids. The redox potential is due to different conditions inside (higher pH, higher Mn2+) and outside (lower pH, lower Mn2+) of the bacterial cell[6][5]. Iso-α-acids or humulinic acids passing into the cell, form complexes with Mn2+ and transfer electrons out of the cell[6]. By targeted molecular modifications Schurr et al. (2015) determined that the Mn oxidative stress-driven antimicrobial effect of iso-α-acids was more important than antimicrobial effect of the ionophore proton transfer discussed above in the overall antimicrobial activity of hops[5].
Bacterial Resistance to Hop Compounds
Due to the multiple mechanisms for hop antimicrobial activity, multiple resistance mechanisms are necessary for a Gram-positive bacterial cell to successfully be hop-tolerant[6]. Hop resistance of bacteria will vary by species as well as within a species with individual strains. The environment in which strains are cultured and maintained may also influence their hop tolerance. The hop tolerance of lactic acid bacteria strains decreases when they are cultured in hop-free environments and strains cultured in media with increasing concentrations of hop compounds show an increase in hop tolerance[4]. the stability of hop resistance, or the rate at which it is lost when bacteria are cultured in unhopped wort, varies by strain. It can take up to 1 year for maximum loss of hop resistance, suggesting that in some strains have a relatively stable hop resistance[4]. Because of this intra-species variability and dependence on how the strains were cultured, it is difficult to give specific advice about the hop-tolerance of a wide range of strains offered from different sources. As a general rule, some common lactic acid bacteria species used in sour beer and found as beer spoilage organisms like Lactobacillus brevis, Lactobacillus lindneri and Pediococcus delbrueckii have some resistance to hops[4]. Brewers seeking to make acidic beers with higher doses of hops may wish to seek out one of these species. Some hop-tolerant species benefit from pre-culturing in media with below-limiting concentrations of compounds before being used in more highly hopped wort or beer[7].
Aged Hops
In lambic brewing, aged hops refers to hops that have been aged for 2+ years in non-refrigerated conditions, and in burlap sacks or some other oxygen permeable bag [8]. It should also be noted that "aged hops" can refer to any sort of hop aging (especially in scientific literature), including short term hop aging (1-6 months, for example) in refrigerated or non-refrigerated temperatures. Much of the information below references hops that have been aged in warm conditions for shorter time periods than what hops are aged for in lambic brewing. The additional aging of hops that are used in lambic brewing or similar beers might have different effects than what has been studied in hops that are aged for shorter periods of time.
Aging hops leads to oxidation of acids and oils. Generally brewers seek to avoid this to preserve the aromatic and bittering properties of their hops; however some beer styles make extensive use of aged hops. Aged hops still retain some antimicrobial properties and can be used for microbial inhibition. In addition to their antimicrobial activity aged hops contribute important flavor and aroma compounds and precursors to beer.
Chemistry and Characteristics
(in progress)
Acids
During aging, both alpha and beta acids oxidize and degrade with warmer temperatures and more oxygen exposure having a greater impact. This corresponds with an increase in the Hop Storage Index (HSI). As the oxidation of hop oils rises, the HSI number on a lot of hops increases [9][10]. These oxidized compounds lead to a higher amount of non-alpha-acid bitterness compounds in aged hops, and have a remarkable effect on the bitterness of beer. The bitterness from oxidized hop compounds has been described as more earthy, harsh, and astringent than the sharper, cleaner bitterness from iso-alpha acids [11].
Storage conditions and variety play a large role in how acid content in hops changes over time. Beta acids are generally more resistant to oxidation than alpha acids. A study by Mikyška and Krofta (2012) found that after 12 months of storage at 20°C in open air, hops lost 64-88% of their alpha acid content and 51-83% of the beta acid content, with the beta acids dropping off more significantly after 6 months (alpha acid content declined steadily throughout the aging period). These amounts varied with different Czech hop varieties (Saaz, Sládek, Premiant, and Agnus), and beta acids degraded slower than alpha acids as seen below [11]:
| Storage [11] | Oil | Saaz | Sládek | Premiant | Agnus |
|---|---|---|---|---|---|
| Open air at 20°C for 12 months | |||||
| Alpha acids | -80% | -88.3% | -64.3% | -78.2% | |
| Beta acids | -60.5% | -83% | -53.7% | -51% | |
| Vacuum sealed at 20°C for 12 months | |||||
| Alpha acids | -20.6% | -24.9% | -22.2% | -21.7% | |
| Beta acids | -2.7% | -1.7% | -2.1% | -1.2% | |
| Vacuum sealed at 2°C for 12 months | |||||
| Alpha acids | -1.1% | -5.5% | -0.3% | -1.4% | |
| Beta acids | -1.7% | -2.3% | -0.4% | -0.5% |
Oxidized alpha acids (humulinones) are similar in taste perception to iso-α-acids, but have been described as less bitter (about 66% as bitter) [2][10]. Humulinone content increases in hops after being pelletized (whole leaf hops have less humulinones). In fresh pellet hops that have a relatively low humulinone content, the humulinones contribute little to the bitterness of the beer when boiled, however when dry hopped they readily dissolve into the beer and have a significant impact on the beer's bitterness. With heavy dry hopping, the humulinones also decrease iso-alpha acid content of beer with more than 20 IBU's, but not in beer with less than 25 IBU. The decrease in iso-alpha acids and perceived bitterness/IBU is partially made up for the bitterness of the humulinones themselves (humulinones are picked up in IBU measurements with a spectrophotometer). In beers with less than 25 IBU, high dry hopping rates greatly increase the bitterness/IBU due to the bitter humulinones. The rate of humulinone formation is limiting, meaning that humulinone formation occurs rapidly during hop pelletization, and the concentration peaks during this time (researchers found that further exposure to air did not increase humulinone content). Scientists believe that this is because when whole leaf hops are baled, only 20% of lupulin glands are broken, whereas when they are pelletized 100% of the lupuline glands are broken. The exact mechanism by which alpha acids are converted to humulinones is not known [10]. Humulinone content in long-aged hops (1+ years) has not been studied.
Oxidized beta acids (hulupones) also contribute to perception of bitterness.
Oils
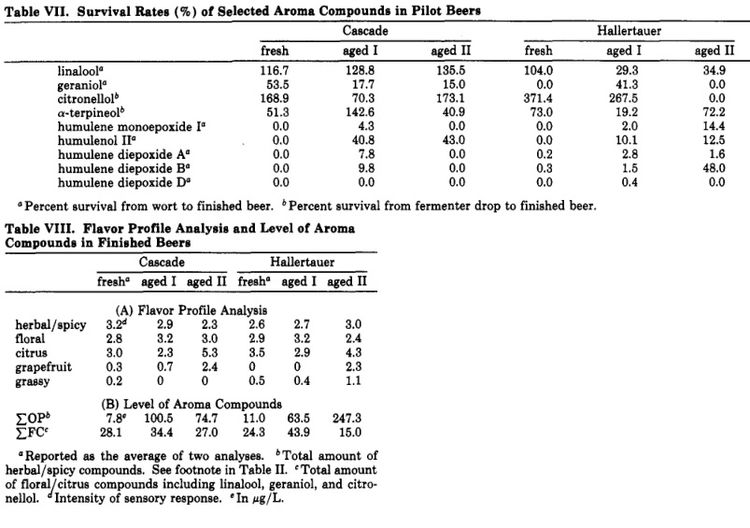
Hop oils also generally degrade over time, however their degradation rates are more complex. Lam et al. (1986) found that aging both cascade and North American grown Hallertauer Mittelfrueh resulted in an increase in grapefruit-like character, although the compound that caused this was not identified. In the case of Cascade the intensity of this flavor correlated with the age of the hops [9]. In the Hallertauer hops, aging resulted in an increase in a spicy/herbal character [9], which is in agreement with reports of oxidized sesquiterpenes (specifically humulenol II, humulene diepoxides, caryophyllene, and to a lesser extent humulene monoepoxides and alpha-humulene) contributing a spicy/herbal flavor to beer [12][11]. Many of the oils followed in the Lam et al. (1986) study which increased during a short accelerated aging period (2 weeks at 90°F) then decreased during extended aging (60 additional days at 90°F). The cascade hops lost more of the fruity/citrusy hop oils (myrecene, linalool, geranial) than Hallertauer, suggesting that different strains of hops can withstand aging better than others. The concentration of hop oils are affected by the brewing process and fermentation (see the table) [9]. Another study found that beta-ionone (classified as a ketone, and characterized as "floral" and "woody" [13]) increased in beers brewed with hops that were aged for 30 days at 40°C versus beers brewed with aged hops [14].
Cheesy oxidation compounds which can be esterfied to form fruity tasting compounds[2].
- "Increasing Bitterness By Dry Hopping", article by Scott Janish on oxidized alpha acids.
- Hulupones - oxidized beta acids.
Esters
During fermentation, it is believed that esters are produced by yeast metabolism from hop compounds such as alpha acids, beta acids, polyphenols, and hydrocarbons because they are not found in unhopped beer or in hops themselves. These esters include ethyl 2-methylpropanoate (citrus, pineapple, sweetness), ethyl 2-methylbutanoate (citrus, apple-like), ethyl 3-methylbutanoate (citrus, sweetness, apple-like), 2-phenylethyl 3-methylbutanoate (floral, minty), and 4-(4-hydroxyphenyl)-2-butanone (citrus, raspberry) [15]. Kishimoto et al. found that some beer esters were increased when using unidentified pellet hops (described in the study only as "a bitter variety of 11.5% alpha acid") that were aged for 30 days at 40°C versus using fresh pellet hops that were stored cold (4°C). Specifically, in the beers that used the aged hops, they found a significant increase in citrus esters (ethyl 2-methylbutanoate, ethyl 3-methylbutanoate, and 4-(4-hydroxyphenyl)-2-butanone), and a decrease in "green, hop-pellet-like, and resinous" compounds such as myrcene and (Z)-3-hexen-1-ol in the beers made from aged hops. The beers brewed with aged hops were described as more citrusy, while the beers brewed with fresh hops were described as more "hop pellet-like", resinous, floral, and "green". The authors speculated that since these esters were not present in beers brewed without hops that they were derived from the humulone and lupulone oils in the hops during yeast fermentation [14].
Thiols
Kishimoto et al. found an increase in the thiol 3-methyl-2-butene-1-thiol (MBT) in beers that were brewed with unidentified pellet hops (described in the study only as "a bitter variety of 11.5% alpha acid") that were aged for 30 days at 40°C versus using fresh pellet hops that were stored cold (4°C). Interestingly, this thiol was higher in beers where the aged hops were added to the boil rather than when they were added after the wort was cooled. The authors were not able to determine whether or not the MBT was derived from yeast fermentation, or from boiling the hops, but aging the hops increased the precursors for MBT [14]. MBT has been described as the thiol that produces the "skunky" aroma in light struck beer [16].
Aged Hops in Lambic
Modern lambic traditionally uses aged hops at a moderate rate to help limit and select for microbes and regulate acid production. Modern Lambic brewers cite rates in the range of roughly 450 grams of hops per Hl of finished beer [17] (~43 min in) (see also the notes pertaining hopping rates on the Cantillon page), with some brewers possibly going above this range. The age of hops used depends on the producer and their preferences/stock. Cantillon uses hops that are on average 2-3 years old (source----), which 3 Fonteinen reports using hops that are over 10 years old[18] (~48 minutes in).
Historic hopping in lambic and other mixed-fermentation beer
While modern lambic uses aged hops almost exclusively, it was common for historic lambic to blend both aged and fresh hops[19]. The exact ratio of fresh to aged hops changed over time and could vary depending on the harvest (poor hop years may have relied more heavily on aged hops while years of good harvests would make more use hops of the recent harvest). In addition to the difference in hop age between modern and historic lambic, hopping rates also differ significantly between modern and aged hops. It is important to note that the quality of these hops are certainly different from modern hops, and that hop origin could have a significant influence on suggested hopping rates [20] (see the hopping rate table and notes regarding hop origin conversion factors from historical texts). While hop quality would have improved moving to the modern day while hopping rates were dropping, there is mention in historic lambic literature of lambic in the late 1800s being more bitter than lambic from the mid 1900s (and, subsequently, similar to historic saison in the increased hop presence in a mixed-fermentation beer)[19]
Historical documents dealing with Belgian brewing show a steady progression from high doses of fresh hops in lambic to the sort of hop composition and origin that are in use today. In 1851 Lacambre mentions rates for Belgian hops of 760-860 g/Hl and specifically highlights the use of young hops. Belgian brewing scientist Henri Van Laer recommended a hopping rate of 700-800 g/Hl in 1890, roughly in agreement with Lacambre though slightly lower. In the early 1900s, citing information from 1896, Le Petit Journal du Brasseur mentions a hopping rate of 540 g/Hl using a mix of Belgian and Bavarian hops and a split of 2/3 young, 1/3 old in good years (and 50/50 in bad years). In 1928 Le Petit Journal du Brasseur recommends a larger proportion of aged hops (2/3 aged, 1/3 fresh) and rates of 600g/Hl of Belgian hops[19]. Considering the difference in strength in German and Belgian hops[20], this fits with a stable or decreasing hopping rate from that given in the early 1900s. In 1937 exclusive use of aged hops is recommended, though as noted in 1946, year old hops may be preferable to hops that were aged longer in poor conditions[19]. Also in the 1940s Le Petit Journal du Brasseur recommends hopping rates of 400-500 g/Hl, roughly in agrement with modern times, and notes that the lambic of this time was softer than historic lambic[19].
(In Progress) Lambics aren't the only historic mixed-fermentation beer to make use of aged hops. Though the specific mention of aged hops for saison and bieres beers does not seem to be the norm, aged hops were used at times, such as when more acidity was desired. These hops were also more likely to be used toward the beginning of the brewing season in months like October where the current harvest may have been considered too fresh for proper use. Notes: Give some discussion of hopping saison and bieres de garde. See hopping grisette table for some hopping rates, PJB, etc.
Aged Hop Suppliers
- Hops Direct "Choice Debittered/Aged Hops" (Leaf - Cascade).
- Hops Direct "Choice Debittered/Aged Hops" (Pellet - Willamette).
- Freshhops "Lambic Hops" (Leaf - Willamette)".
- Yakima Valley Hops "Lambic / Aged Hops" (Pellet).
- Farmhouse Brewing Supply "Lambic Hop Blend" (Pellet - Blend of varieties).
- The Malt Miller (UK).
- Brew Store UK (Leaf - Fuggles).
- Brew Store UK (Leaf - Hallertau).
Glycosides and Brettanomyces
Hops contain glycosides, which are flavor compounds that are bound to a sugar molecule. In their bound form, glycosides are flavorless. Enzymatic activity from some strains of Brettanomyces can release these bound compounds and release their flavor and aromatic potential. See the Glycosides page for details.
Techniques
(In progress)
Kettle Hopping
Kettle hopping sour beers can be a difficult thing for the new sour beer brewer. The usage of hops generally inhibits most lactic acid bacteria species, however there are many exceptions to this. Lactic acid bacteria can have a range of hop tolerance, with species such as Lactobacillus acetotolerans that tolerated Goose Island's Bourbon County Stout at 60 IBU [21]. Some breweries report that their house lactic acid bacteria can tolerate IBU ranges up to 10-20 IBU. White Labs claims that their L. delbuekii (WLP677) is tolerant of up to 20 IBU, however most Lactobacillus cultures from yeast labs are not hop tolerant [22]. See the Lactobacillus culture charts and hop tolerance for more information.
For both mixed fermentation sour beers and kettle sour beers, hops are often not used at all. In the case of kettle sours, sometimes brewers opt to add hops after the wort has been soured (see Wort Souring). For lactic acid cultures that are hop tolerant, hops can be used as a way to inhibit the amount of acid produced by them if the brewers desires this.
A popular technique for 100% Brettanomyces Fermentation is to use a typical IPA recipe. Hops do not inhibit Brettanomyces yeast. Some of the fruity characteristics of Brettanomyces can compliment the fruity character of hops such as citra, amarillo, and galaxy. For beers that are fermented with just S. cerevisiae and Brettanomyces but not lactic acid bacteria (such as some American farmhouse ales), Old World and noble hops are often used as well as North American and New Zealand/Australian citrusy hops.
Dry Hopping
Brewers have had positive and interesting results dry hopping sour and funky beer. Often fresh American or New Zealand varieties that compliment fruit flavors are chosen, however other varieties have been used as well, including English and German hops. Just as in dry hopping normal beers, dry hopping sour/funky should be done after the beer has matured. Dry hopping for around 1-3 days is adequate for extraction, depending on whether or not the beer is recirculated or agitated (agitation of the beer while on contact with the dry hops attains full extraction in 24 hours) [23].
Dry hopping can contribute to bitterness in beer through oxidized alpha acids and oxidized beta acids. Dry hopping also has a linear impact on the pH of beer regardless of the starting IBU or pH: the pH rises by 0.14 per pound of hop pellets per barrel of beer (~0.5 ounces per gallon) [10][2].
- MTF thread on using very small amounts of subtle effects.
- Caroline Whalen Taggart's data point on the effects of dry hopping on L. plantarum (GoodBelly). No hops finalized at a pH of 3.53, and the dry hopped version finalized at a pH of 4.35.
- Per Buer's experiment on the effects of dry hopping on Lactobacillus:
See Also
Additional Articles on MTF Wiki
External Resources
- Per Buer's Video Demonstration of how dry hopping inhibits Lactobacillus.
- Blog Article on Brett and Glycosides by Cy Wood.
- How hops prevent infection, by Lars Garshol.
- Unlocking Hop and Fruit Flavors from Glycosides, HBT article by Dennis L Waldron.
- Beer Legends Hop Varieties - gives vital statistics on hops including acid content, physical cone characteristics, storage and growth details, and oil content.
- TTB Hop Requirements for USA commercial breweries.
- Blog article on hops in spontaneous fermentation by Dave Janssen
References
- ↑ 1.0 1.1 Schönberger and Kostelecky, 2012
- ↑ 2.0 2.1 2.2 2.3 2.4 Shellhammer, Vollmer, and Sharp. Oral presentation at the Craft Brewers Conference, 2015.
- ↑ 3.0 3.1 Fernandez and Simpson (1993)
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Sakamoto and Konings (2003)
- ↑ 5.0 5.1 5.2 Schurr et al., (2015)
- ↑ 6.0 6.1 6.2 Behr and Vogel, (2010)
- ↑ Simpson and Fernandez, 1992
- ↑ "Home for Our Aged Hops". Jester King's blog. Retrieved 11/18/2016.
- ↑ 9.0 9.1 9.2 9.3 Aging of Hops and Their Contribution to Beer Flavor. Lam et al. 1986.
- ↑ 10.0 10.1 10.2 10.3 Humulinone Formation in Hops and Hop Pellets and Its Implications for Dry Hopped Beers. John Paul Maye, Robert Smith, and Jeremy Leker. 2016.
- ↑ 11.0 11.1 11.2 11.3 Assessment of changes in hop resins and polyphenols during long-term storage. Alexandr Mikyška and Karel Krofta. 2012.
- ↑ Goiris et al., 2002
- ↑ Beta-ionone. Good Scents Company. Retrieved 11/22/2016.
- ↑ 14.0 14.1 14.2 Odorants comprising hop aroma of beer: hop-derived odorants increased in the beer hopped with aged hops. Toru KISHIMOTO , Katsunori Kono , Kenkichi Aoki. 2007.
- ↑ Comparison of the Odor-Active Compounds in Unhopped Beer and Beers Hopped with Different Hop Varieties. Toru Kishimoto, Akira Wanikawa, Katsunori Kono, and Kazunori Shibata. 2006.
- ↑ 3-methyl-2-butene-1-thiol. Aroxa website. Retrieved 11/22/2016.
- ↑ Jean van Roy on Basic Brewing Radio
- ↑ Drie Fonteinen on Belgian Smaak
- ↑ 19.0 19.1 19.2 19.3 19.4 Dave Janssen's discussion of hopping in spontaneous fermentation
- ↑ 20.0 20.1 Dave Janssen's discussion of hopping grisettes
- ↑ MTF thread that reported an MBAA presentation by Brett Porter from Goose Island. 07/30/2016.
- ↑ "Commercial Brettanomyces, Lactobacillus, and Pediococcus Descriptions; Commercial Yeast Laboratories." The Mad Fermentationist blog. Michael Tonsmeire. Retrieved 12/12/2016.
- ↑ A Study of Factors Affecting the Extraction of Flavor When Dry Hopping Beer (master thesis). Peter Harold Wolfe. 2012.

