Tetrahydropyridine
Tetrahydropyridines are a group of flavor active compounds found in some wines and sour beers. While harmless, they are often considered to be off-flavors in wine and beer. With the recent increase in spontaneously fermented wine and lower sulphite usage by certain wine producers, these flavors have become more prevalent in wine [1]. Forms of tetrahydropyridines (abbreviated and often referred to generically by brewers and winemakers as THP), specifically 2-acetyl-3,4,5,6-tetrahydropyridine and 2-acetyl-1,4,5,6-tetrahydropyridine (abbreviated ATHP or ACTPY), 2-ethyltetrahydropyridine (abbreviated ETHP or ETPY), and 2-acetylpyrroline (abbreviated ACPY or APY) [2], which are classified as a ketone and a cyclic imine [3]. They are commonly attributed to the "mousy", "urine" (in high amounts) Cheerios® or Cap'n Crunch® (in low amounts), "breakfast cereal", or more generically, "cracker biscuit" flavor in sour beers and wine. The flavor is detected towards the end of the swallow, and the aftertaste can remain for more than 10 minutes [4]. 30% of winemakers are not able to detect the flavor of THP in wine (we do not know if this statistic is reflected in sour beer, but some people have reported not being able to taste THP in sour beer) [5]. The low pH of sour beer or wine makes it harder to detect the flavor and often impossible to detect the aroma. An increase in pH is needed in order to detect the flavor of THP, and the mouth's salivary pH serves that purpose when tasting beer or wine with THP. For example, as the mouth's pH adjusts back up after swallowing a sip of sour beer or wine, the THP becomes detectable in the aftertaste. This effect on sensory detection by low pH might also explain why some people are better at detecting it since people have different pH's on the surface of their tongues and saliva [2]. Diacetyl is sometimes mistakenly indicated as a potential cause of "mousy" flavors in sour beers, however, tetrahydropyridines are the accepted cause. The flavor tends to age out of sour beers after 2-6 months in the fermenter, kegs, or bottles (although aging periods as long as possibly 8-12 months have been reported on MTF [6]). The exact mechanism for how THP ages out of beer is not fully understood, and it is unknown whether cold or room temperature storage speeds up the breakdown process. It is more likely that room temperature storage will result in faster breakdown of THP, and anecdotes from MTF members seem to support this [7][8][9][10]. Many brewers have noticed that pitching rehydrated wine yeast at packaging reduces the amount/duration of this flavor in kegs and bottles [11].
Traditionally, the mousy/Cheerios® flavor from THP is considered an off flavor in both wine and sour beer. There is some debate and differing opinions as to whether or not a small amount of THP flavor is allowable (or even enjoyable) in sour beers, however most consider any level to be an off flavor [12]. In wine, THP is also generally considered an off-flavor, although to some people a small amount of THP is acceptable in natural wine. It has greatly been eliminated as a problem in wine making due to sulphite usage and better controlled fermentations. However, in recent years there has been an increase in the popularity of natural wine which has a higher chance of being affected by THP. There has therefore also been a recent increased attention to THP in wine due to the increased popularity of natural wine [13].
In food, Tetrahydropyridines are associated with the aroma of baked goods such as white bread, popcorn, and tortillas, and is formed by Maillard reactions during heating. These versions of THP have a different chemical form than the forms produced by microbes, but they can have a similar flavor.
Contents
History of Scientific Research
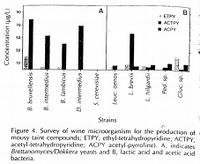
THP in wine ("mouse taint") was first described in wine by Müller-Thurgau and Osterwalder in 1913, although mention of an off-flavor in wine that 'is closely resembling the smell of a residence of mice' dates to 1894 in "A Treatise on wines" by J.L.W. Thudichum. Müller-Thurgau and Osterwalder attributed it to be produced by bacteria. They also established that the ability to detect THP varies from person to person [14]. A 1908 text by the Germans Nessler and Windish linked THP off-flavors to wine that aged longer on lees, perhaps due to yeast autolysis [2]. In the 1940's, some scientists proposed that THP was purely a chemical reaction, however, their evidence was inconclusive. In 1977, Tucknott et al. was able to identify that THP production was attributed to both Brettanomyces yeast and lactic acid bacteria, and that ethanol and L-lysine played a role in its production, and this was confirmed by Heresztyn et al. in 1986 [14].
The chemical analysis of THP has proven to be a difficult endeavor in science. In 1995, Herderich et al. out of Australia developed a method for chemically analyzing THP for the first time. It was at this time that all three forms of THP (ETHP, ATHP, and APY) could be identified consistently in contaminated wine. It was also during this time that the three forms of THP contributed to the flavor of various foods, such as tortilla chips and taco shells. For example, APY was found in the fermentation of cocoa in 1995. In 1995, Grbin et al. described Brettanomyces yeast as the yeast that produces THP, while wine strains of S. cerevisiae did not [14].
In 2000, the Australians Grbin and Henschke showed that some strains but not all of B. bruxellensis, B. anomalus, B. nardenensis, and B custersianus produce THP, and that THP production was influenced by the carbon source, but not dependent on it (THP was still produced in dry wines with little available nutrients, and fermentation rate was not always related to THP levels) [15].
In 2007, the Australian team of Grbin et al. confirmed that higher lysine levels increased the amount of ATHP produced (but not ETHP), although with diminishing increases of THP as the lysine level was increased. They also discovered that L-ornithine also functioned as a precursor for THP production in Brettanomyces. The group proposed a biochemical pathway for the different forms of THP in Brettanomyces [16].
Detection Methods
In 2007, Grbin et al. developed a complex and unique method of analyzing forms of THP using a Finnigan TSQ 70 mass spectrometer directly coupled to a Varian 3400 gas chromatograph. The chromatograph was equipped with a 30 m J&W Carbowax 20 CAM fused silica column, 0.25 mm i.d, and 0.25 μm film thickness [16]. More recently, Hayasaka (2018) developed a reliable and rapid but pricey method to detect THP in wine using specialized HPLC-APCI-MS/MS equipment [17].
Due to the specialized GC/MS equipment needed for measuring forms of THP that most labs do not have, other human sensory-based methods have been developed in the winemaking world. Originally, a "palm & sniff" method was developed to detect THP where a small amount of wine is rubbed on the palm which increases the pH of the wine and then immediately sniffed to detect THP. Since then, some studies have used alkaline strips as a way to smell the aroma of THP, and while not as precise as the specialized GC/MS lab equipment, could still help further the understanding of THP. Knowing that mousy off-flavor has a lingering sensory impact, the technique of alkaline paper strip assessment was adapted from Heresztyn (1986a). Paper strips (Whatman No. 1, 4–5 mm × 50 mm) were prepared by soaking in NaOH (0.1M) and drying overnight at room temperature. The alkaline paper strips were then briefly dipped into cell-free samples (centrifuged) and immediately assessed for the mouse-like odor by sniffing [18].
Tempère et al. (2019) developed what they suggest is a better way to test for mousy off-flavor in wine via oral sensory, specifically as a way to enable panelists who are not as sensitive to THP to detect it during sensory testing. They compared the alkaline strips method to a method where the wine's pH is increased by adding sodium bicarbonate to a pH of 5 and a pH of 7. This mild base is contained in human saliva. At a pH of 5, sensory panelists were more easily able to detect APY and to correctly order the intensity of APY in wine than when they used alkaline strips. For example, the range of detection level for all panelists went from a range of 15 - 300 µg/L to a range of 0.3 - 30 µg/L. At a pH of 7, panelists were not as easily able to detect the aroma of APY. Keep in mind that this test does not reflect the real world tasting of wine since the pH would never be raised during normal consumption, but it could be used by a sensory program as a way to more reliably detect smaller amounts of APY in wine [19].
Kiyomichi et al. (2023) developed a simple method to accurately detect ATHP, ETHP, and APY using gas chromatography-mass spectrometry with stir bar sorptive extraction (SBSE-GC–MS instrumentation). Thermal desorption and injection were performed using a Twister thermal desorption unit (TDU) and a Gerstel CIS 4 cooled injection system with a programmable temperature vaporization (PTV) inlet, installed on an Agilent 6890 gas chromatograph combined with an Agilent 5975 Mass Selective Detector (Agilent Technologies, Massy, France), equipped with a Gerstel MPS 2 autosampler (Gerstel, Mülheim an der Ruhr, Germany). An HP-5MS fused-silica capillary column (30 m × 0.25 mm, 0.25 µm, film thickness, SGE, Courtaboeuf, France) was used, with helium as carrier gas (Messer France S.A.S, Suresnes, France) at a constant pressure of 70 kPa, corresponding to an initial flow of 1.3 mL.min−1 [20]. See also "Simultaneous assay of mousy off-flavor markers in wine," Ives Technical Review.
Liesel Krout, Lucille Benedict, Meg Hausman, and Zach Bodah from University of Southern Maine and Allagash Brewing Company have begun developing a new way to produce an ATHP flavor spike and detection method. See the poster for more details. This method is still in development.
In 2024, UC Davis described their method for detecting THP in beer for the first time. The team used Liquid Chromatography Mass Spectrometry with Electrospray Ionization (LC-MS-ESI). Extraction of ATHP in the samples was performed using QuEChERS (quick, easy, cheap, effective, rugged, and safe) technique [21]. See also MBAA podcast episode 326, "THP" with Paulina Streimikyte and Glen Fox.
Other techniques for detected THP in food have been developed, which might be applicable to wine or beer. For example, Grimm et al. (2001) developed a technique for detecting 2-acetyl pyrroline (APY or 2AP) in rice. The rice samples had to be heated to 80-85°C in order to extract the volatile APY, and then APY levels in the headspace of the rice container could be detected using solid phase microextraction (SPME) with fibers that operate at the higher temperatures [22]. It isn't known if such methods would also work for measuring THP compounds in beer or wine, but they could provide a potential option for beer and wine researchers. Cider makers have used a baking soda in water solution to help detect THP. Dissolve a small amount of baking soda in water, swish the solution in your mouth for a few seconds, and then spit it out. While the pH of the saliva in your mouth is raised from the baking soda solution, taste the beer/wine/cider to more easily detect THP [23].
The effectiveness of these methods in beer has not been reported to our knowledge (please report any research or anecdotes in the MTF Facebook group).
Forms of THP
ATHP
2-acetyl-3,4,5,6-tetrahydropyridine and 2-acetyl-1,4,5,6-tetrahydropyridine (abbreviated: ATHP or ACTPY) has a much lower flavor threshold than ETHP (see Thresholds), and has been historically cited as the cause of mousy off-flavors detected in wine. In wine, its aroma cannot be detected due to the low pH of wine (it can be detected if the pH is raised), only the flavor. It is easier to detect in higher pH wines. ATHP is the form of THP that is the major contributor to the aroma of freshly baked bread, corn tortilla chips, and crackers. How different foods/wines/beers interact with ATHP on the palate may explain the different flavors that are detected by people, as well differing concentrations and peoples' ability to detect ATHP [2]. In food, ATHP has a slightly different form (6-Acetyl-1,2,3,4-tetrahydropyridin). Its formation in food is due to cooking and thought to be associated with Maillard reactions. This form has a toasty flavor of crackers and popcorn, and its presence disappears quickly in food due to its extreme volatility [24].
ETHP
2-ethyl tetrahydropyridine (abbreviated: ETHP or ETPY) was first identified in wine in 1973, but until recently further studies weren't able to confirm its presence in wine. Its odor threshold is quite high (see Thresholds), and so it was not considered a major source of mousy off-flavors in wine for some time. Consequently, research on ETHP has been limited. More recently, it was shown that Lactic Acid Bacteria (LAB) can produce above threshold levels of ETHP, making it recently important to wine researchers [2].
It has been speculated by scientists studying mousy off-flavors in wine that its production is the result of slow metabolism of ATHP into ETHP by Brettanomyces. ETHP was observed to form much slower than ATHP and coincided with a decrease in ATHP. This slow production of ETHP may be another reason it has been underestimated by researchers until recently [2]. It is not known whether or not ETHP can degrade into another byproduct.
APY
2-acetyl pyrroline (abbreviated: APY, ACPY, or AP) is a more volatile but more potent form of THP. It has a significantly stronger odor and lower odor threshold in wine than ATHP. It can also be found in damp pearl millet, toasted bread, taco shells, tortilla chips, boiled potatoes, cooked sweet corn products, roasted sesame seeds, pan-fired green teas, cured tobacco leaves, peanut and pumpkin seed oils, honey, several soy-based products, and more aromatic rice such as Indian Basmati, as well as many other foods. APY has also been detected in a pale lager beer from Bavaria [25]. APY from microbial metabolism is primarily produced by heterofermentative LAB (see below). In food, APY formation is due to cooking and thought to be associated with Maillard reactions, and its presence ages out quickly in food. For example, Schieberle (1989) showed that heating up yeast and sucrose produced APY, simulating how APY could be produced during baking bread [26]. It is extremely volatile; so much so that the food industry has created powdered forms of APY to increase the flavor stability of some foods associated with it. Some plants such as rice crops, Pandan leaves (Pandanus amaryllifolius), "bread flowers" (Vallaris glabra), Myabi muskmelon fruit, chempedak fruit and jackfruit contain varying levels of APY naturally [2][14][24][27].
Unidentified "Transient" Forms
There have been anecdotal reports of other forms of mousy off-flavors. During the growth of lactic acid bacteria (LAB), mousy off-flavor detection fluctuated with high levels detected early on, and lower levels detected towards the end of growth. This indicates that there may be a transient, strain-dependent form of THP that can occur during malolactic fermentation. There have also been a sensory detection of mousy off-flavors at different levels than the documented levels of ATHP, ETHP, and APY, which were not associated with LAB or Brettanomyces [2].
Sulfur-Containing Forms
Sulfur-containing versions of THP exist in many vegetables and processed meats and are important aspects of their aromas. These include 2-Acetyl-2-thiazoline (the sulfur containing version of APY) and 5-Acetyl-2,3-dihydro-4 H -1,4-thiazine (the sulfur containing version of 6ATHP). These are formed in meat during processing, for example in beef and chicken broths, roasted beef, etc. They are characterized as being similar to APY and ATHP, and have a "roasty", popcorn-like odor and low thresholds. These are chemically formed during cooking and food processing and are not associated with biological metabolism or fermentation [24].
Similar Compounds
A variety of pyridine and pyrazine derived compounds are formed in malt (and other foods) during the malting process as a result of Maillard reactions and have been found to be major contributors to the "malty" flavor of beer. Examples of these compounds include 2-acetylpyridine, 3-acetylpyridine, methylpyrazine, forms of dimethylpyrazine, and trimethlpyrazine. These compounds have a range of flavor descriptors such as creamy, cardboard, grainy, and burnt sugar. For example, 2-acetylpyridine (2AP), also known as 1-pyridin-2-ylethanone, is described as having a malty-biscuity, corn-chip, corn tortilla, or popcorn flavor. These compounds (in particular 2AP) can easily be confused with forms of THP, but they are not the same as the varieties of THP explained above [24][28][29][30][31]. Another form is 2-formyl-1,4,5,6-tetrahydropyridine (FTHP), however, its presence in fermented beverages has not been well studied and it is less stable than other forms of THP, making it difficult to study [32]. One indicator that a particular flavor might be THP instead of one of these malt-derived flavors is that THP is mostly detected on the aftertaste after swallowing, whereas these compounds are often detected during the swallow (although they can sometimes also be detected during the aftertaste).
See also:
- "Flavors From Malt" by Scott Bickham on MoreBeer.com.
- "A pretty corny post" on the "Beer Sensory Science" blog (explains how 2AP could also be confused with DMS).
Production

It is thought that THP in mousy wines/beers is mostly produced by microorganisms. All species of Brettanomyces can produce forms of tetrahydropyridine in varying amounts, although some below threshold. Additionally, Lactic Acid Bacteria (LAB) including Lactobacillus and Pediococcus can produce forms of THP. Acetic Acid Bactera (AAB) has also been demonstrated to produce forms of THP [2][15].
Moulis et al. (2023) studied THP production by 22 strains of Brettanomyces bruxellensis, 20 strains of Oenococcus oeni and 10 strains of Lentilactobacillus hilgardii (formerly classified as Lactobacillus hilgardii), all of which have been reported to produce THP compounds. They found that all strains could produce ATHP, but not all strains could produce ETHP or APY. This variability was determined mostly by species, but also by strain. for example, all of the 22 B. bruxellensis strains only produced ATHP and ETHP and not APY. Variability between strains was less pronounced for the species L. hilgardii compared to the B. bruxellensis and O. oeni strains (different strains of B. bruxellensis, for example, produced much different levels of ATHP/ETHP, where as every strain of L. hilgardii produced relatively the same amount of APY). The researchers also noted that repeatability of THP levels was difficult to achieve, and they owed this to unknown variables such as the physiological state of the cells at time of inoculation into the test media. Interestingly, there was no correlation between strain genealogy and how much THP they produced. The researchers also isolated other species from 25 French wines with mouse taint, including S. cerevisiae, Pichia manshurica, Priceomyces carsonii, Pediococcus parvulus, but none of these strains produced THP in the test growth media [1].
Brettanomyces
Although the exact pathway is not known for Brettanomyces (several are proposed), the conditions for THP production are well documented. ATHP is produced by metabolizing the amino acid L-lysine or D-lysine [16], along with ethanol and a glucose or fructose molecule. Iron is also needed for THP production, although its exact role in biosynthesis is not known [2]. As with other amino acids, lysine is taken up by Saccharomyces during fermentation and then released after fermentation. Levels of lysine fluctuate slightly throughout fermentation but are generally high throughout a beer's lifetime (including after fermentation) [34][35][36][37]. Wheat generally has a slightly lower amount of lysine than barley, and oats have a slightly higher amount of lysine than barley [38][39][40][41]. In red wine, yeast autolysis releases many amino acids including lysine [42][43][44]. Aging beer on trub and its effects on THP production has not been studied, but it might not be a factor in beer since lysine levels are high in beer regardless of yeast autolysis [45].
Oxygen plays a key role and has a stimulatory effect in ATHP and ETHP production (particularly ATHP), but its exact role is not understood. It has been speculated that since ATHP production is associated with Brettanomyces growth, and Brettanomyces grows better under aerobic conditions, that this is why more ATHP is produced under aerobic conditions [46][16][47]. It has also been hypothesized that oxygen may have a direct effect on the THP molecules themselves [2]. ATHP production was also shown to increase when anaerobically precultured cells were transferred to an aerobic environment, indicating that oxygen has a direct role on the production of ATHP, not just a byproduct of Brettanomyces growth [2]. Limiting oxygen exposure during kegging/force carbonating is recommended for helping to reduce ATHP production; even very small amounts can have an effect (although the exact threshold of how much oxygen is required has not been determined). For example, the purity of the CO2 supply should thus be taken into consideration when force carbonating. At 0.5% impurity (the impurity is air, 1/5 of which is oxygen) and at 2 volumes of CO2, ~1,420 ppb of O2 would be added to the packaged beer, which is an exceedingly high amount of oxygen. The CO2 supply should ideally be 99.990% pure or better (this would introduce 46 ppb of oxygen at 2 volumes of CO2). The method that the CO2 is added can also determine how much oxygen is introduced into the packaged beer. Sparging CO2 (bubbling it through the beer) dissolves significantly less oxygen due to Henry's Law (see reference), while injecting (flushing) dissolves significantly more oxygen [48][49]. Vessel purging methods with CO2 are also less efficient than some might expect, and might still leave enough oxygen behind to stimulate THP production (see this HomebrewTalk thread). Pitching fresh Saccharomyces at bottling/kegging time and naturally carbonating the beer with sugar has reportedly reduced mousy off-flavor detection, perhaps because Saccharomyces metabolizes both the oxygen and sugar faster than Brettanomyces. Different strains of S. cerevisiae might be more efficient than others at helping reduce THP. For example, Mitch Ermatinger from Speciation Artisan Ales anecdotally observed that switching from CBC1 conditioning yeast to EC1118 reduced THP off-flavors from 1 month to two weeks [50] (see Packaging for details on re-yeasting at packaging time). Justin Amaral and Mike Karnowski reported an anecdote that purging bottles with CO2 reduced THP levels, although limiting oxygen in the bottle also had some negative effects on some conditioning yeast strains [51].
Interestingly, for unknown reasons Brettanomyces cells grown under aerobic conditions and then transferred to an anaerobic environment still produced significant amounts of ATHP in the anaerobic environment. It has been suggested that the aerobic conditions made the Brettanomyces cells predisposed to creating ATHP [2]. Oxygen exposure during Brettanomyces starters could potentially stimulate ATHP production later on down the road, even if the beer is not exposed to oxygen, although anecdotal evidence shows that this may not be a concern for brewers. It is still advised to use an aerobic or semi-aerobic starter for Brettanomyces unless the brewer believes this might be the direct cause of ATHP problems in their beer because Brettanomyces requires at least a small amount of oxygen for growth. Any other oxygen pick up after the beer has finished fermentation is the more likely cause of THP production and the brewer's post-fermentation processes should be examined first.
The level of ATHP production varies widely between species and strains of Brettanomyces, with some strains producing insignificant amounts and others producing very high amounts above taste threshold [15]. Additionally, ATHP production requires glucose or fructose, which explains why ATHP may be seen more often in stuck wine fermentations rather than wine that has finished fermenting. ATHP production by Brettanomyces was observed in wine with glucose or fructose added, along with synthetic growth media, suggesting that the type of growth substrate does not effect production [52].
The production of ATHP is not efficient, meaning that the amount of ATHP produced is not proportional to the amount of L-lysine consumed. Therefore, the production of ATHP appears to be a byproduct (secondary metabolite) of L-lysine catabolism [2]. ATHP is further metabolized into ETHP by Brettanomyces, although not much is known about this metabolic process [53][2]. ETHP has a significantly higher taste threshold, and is often not detected in contaminated wine [47].
Although Brettanomyces is capable of producing APY from L-ornithine [16], the amount produced is much less than that of LAB and high amounts of L-ornithine are required. In wine, there isn't enough L-ornithine present to production significant amounts of APY from L-ornithine. Therefore, the presence of APY (which is much easier to detect aromatically than ATHP) indicates a bacterial contamination in wine (it is unknown if this applies to beer) [2]. Additionally, Moulis et al. (2023) found that out of 25 French wines with THP, only 20% of them had B. bruxellensis in them, indicating that THP is mostly produced by bacteria or chemically in wine [1].
The presence of the "mousy off-flavor" caused by forms of THP appears to be temporary in beer. Although not much is known about the degradation or metabolic breakdown of ATHP/ETHP, it tends to age out of beer after 2-6 months. Since the odor/taste threshold for ETHP is much higher than ATHP, and ATHP appears to be metabolized into ETHP by Brettanomyces over time, this may be one of the mechanisms by which the mousy off-flavor ages out of beer. The possibility of ETHP breakdown is not mentioned in any studies that we know of, although Moulis et al. (2023) reported that for organisms that produced ETHP, there was always a 1:10 ratio between ETHP/ATHP or ETHP/APY, suggesting that this ratio might be governed by the chemistry of the media used and/or the reduction potential [1]. This was confirmed by second study by Moulis when B. bruxellensis was co-fermented or not co-fermented with APY-producing strains of Pediococcus paravulus [54]. Another unknown is why does Brettanomyces produce ATHP shortly after kegging and force carbonating a beer that has reached final gravity. The most likely cause is oxygen pick up during the kegging process. Pitching fresh Saccharomyces at bottling/kegging time and naturally carbonating the beer with sugar has reportedly reduced mousy off-flavor detection, perhaps because Saccharomyces metabolizes both the oxygen and sugar faster than Brettanomyces.
Co-fermentation with LAB
Moulis et al (2024) published a second study where they measured ATHP, ETHP, and APY produced by three ATHP/ETHP-producing strains of B. bruxellensis and three strains of APY-producing Pediococcus parvulus. They measured levels of these three THP compounds when one of each B. bruxellensis was co-fermented with one each of the strains of P. parvulus. They compared these levels to when each pair (one strain of B. bruxellensis and one strain of P. parvulus) were fermented on their own, and then summed the total levels of each THP compound between the two separate fermentations. They found that APY levels, which was only produced by the P. parvulus' strains and not the B. bruxellensis strains, were much lower if the strains were co-fermented with any of the three strains of B. bruxellensis'. However, for ATHP and ETHP, 2 of the 3 strains of B. bruxellensis produced different levels of ATHP or ETHP when P. parvulus was co-fermented with them. The strain of P. parvulus also had an impact on this co-fermentation; some combinations of co-ferments produced more ATHP but less ETHP. The study concluded that the impact of co-fermentation inhibits APY produced by bacteria despite the strains used, and it can have an inhibitory or stimulatory impact on ATHP/ETHP, depending on the combinations of strains. The authors suggested several possible explanations [54]:
Previous results (Strickland et al., 2016) showed that less 4-ethylphenol (EP), a compound contributing to the “Brett character” in wine, was produced when P. parvulus and B. bruxellensis were jointly inoculated rather than separately. Here, P. parvulus and B. bruxellensis produce more ATHP and no APY at all when they are together. There are several hypotheses to explain that. First, Moulis (Moulis, 2023), proposed that ATHP production could correspond to a signal or a response to a signal between the different cells of B. bruxellensis, to respond to stress. Here, B. bruxellensis could reply to the stress of the presence of P. parvulus by ATHP over-production. P. parvulus could thus directly influence the metabolism of B. bruxellensis, resulting in the production of more ATHP than 4-ethylphenol in its presence. A second hypothesis is that B. bruxellensis could change the balance of acylation by P. parvulus. Costello and Henschke (2002) proposed a formation pathway for APY and ATHP by LAB from ornithine and lysine, respectively. This formation pathway, in both cases, ends up with acylation (of piperideine for ATHP and of pyrroline for APY). B. bruxellensis could inhibit APY production either by inhibiting the pathway or by preferentially inducing ATHP acylation by P. parvulus. Moreover, one of the production routes proposed by Grbin et al. (2007) involves cadaverine as a biosynthetic intermediate. Lactic acid bacteria such as O. oeni or P. parvulus are known to produce large quantities of biogenic amines (Granchi et al., 2005; Wade et al., 2019) and they can biosynthesise cadaverine to be metabolised by B. bruxellensis. It would be interesting to explore these hypotheses in a future study [54].
Moulis et al (2024) also attempted to study the effects of co-fermenting B. bruxellensis with Saccharomyces cerevisiae; however, the definitive results were not presented due to a technical issue with the equipment used to measure THP compounds. Nonetheless, the authors suggested that ATHP and ETHP appeared to be higher when B. bruxellensis was co-fermented with S. cerevisiae versus when fermented on its own. The authors hypothesized that this could be due to more acetaldehyde production by S. cerevisiae, which has been identified as a precursor to ATHP production. Another possibility would be nutrients released from less produced by S. cerevisiae [54].
Lactic Acid Bacteria
Heterofermentative Lactobacillus spp., particularly L. hilgardii (reclassified as Lentilactobacillus hilgardii) and L. brevis (reclassified as Levilactobacillus brevis), as well as Leuconostoc oeni [14], can also produce high levels of ATHP, and to a lesser extent APY and ETHP from L-lysine/L-ornithine, ethanol (must be present), and iron. Although many strains of heterofermentative lactic acid bacteria can produce THP, not all do. For example, Costello et al (2008) found that all strains tested of L. brevis (3 strains tested), L. bucherni (3 strains tested), and L.hilgardii (8 strains tested) produced THP, several heterofermentative species did not produce any detectable levels of THP in a grape-based media (one strain each of L. fermentum and L. cellobiosus). Some strains within a species produce high amounts while others produce low amounts, for example Costell et al. (2008) found that some strains of O. oeni produced very high amounts between 50-150 µg/L while others produced very little between 5-20 µg/L in a grape-based media [55]. A strain of L. plantarum (L11a) was shown to produce relatively low amounts. L-lysine stimulates production of ATHP, and L-ornithine stimulates the production of APY [56][57][55][58][59][60]. Acetaldehyde has a stimulatory effect on ATHP and APY production, but is not required. No studies have been done to show whether or not oxygen plays a role in ATHP/APY production in LAB [2]. Most species of Pediococcus do not create forms of THP, although a few species do produce relatively small amounts. In particular, these include P. pentosaceus [61][62], and P. clausenii [63], although one study found no THP in two strains of P. pentocaseus and only transient/occasional THP production in one out of five strains of P. parvulus [56]. Oenococcus oeni and Leuconostoc mesenteroides have also been associated with creating ATHP, APY, and ETHP all above threshold amounts. Since only heterofermentative species produce significant amounts of THP, it is thought that its production is linked to the heterolactic pathway, and thus the metabolism of sugars in LAB [56]. A pathway for APY and ATHP production in Lactobacillus hilgardii was proposed by Costello and Henschke, which involves the intake of lysine or ornithine, along with ethanol (which is broken down into acetaldehyde) to produce APY and ATHP [56]. Lactobacillus pontis has been shown to break down proteins via proteolysis, yielding free amino acids such as ornithine which could serve as precursors to THP formation, and it might be reasonable to presume that other species of Lactobacillus could also free up ornithine as a precursor to THP [24].
Acetic Acid Bacteria and Mould
Although research is limited, acetic acid bacteria (Gluconobacter sp. and many strains of Acetobacter aceti) have been shown to produce forms of THP [2]. Mediterranean dried sauses covered in mould have been characterized as having APY as a flavor contributor. The source of the APY was identified with a mould that grows on the surface called Peniciilium nalgiovense [24]. Moulis et al. (2023) identified several strains of acetic acid bacteria in 32% of selected French wines with mouse taint, but none of the strains produced THP when tested individually in model medium [1].
Impact from Saccharomyces
While S. cerevisiae does not produce THP compounds itself, there is some preliminary data that suggests that the strain of S. cerevisiae could impact ATHP and ETHP levels. Data from the Moulis et al. (2023) study shows that ATHP levels different slightly depending on which strain of S. cerevisiae was co-inoculated with Brettanomnyces. The authors hypothesize that differing levels of acetaldehyde or build up of lees may be the reason different strains of S. cerevisiae might have an impact [64].
Maillard Reactions
It's been shown that various THP compounds can be produced from heat reactions. For example, heating proline with monosaccharides produces a small amount of APY, as does the heating of yeast and sucrose. Phosphate ions are high contributors to THP production via heat reactions, with the amino acids proline, ornithine, and citrulline being precursors (the first two of which are important amino acids in yeast), and 1-pyrroline being the intermediary step. Ornithine is the precursor to heat produced ATHP in bread making [65][24]. 2-Acetyl-1-pyrroline forms from Maillard reactions and is key to the aroma of cooked Lipu taro [66]. These reactions mostly occur at a relatively basic pH (7-9) [67].
Oxygen Reactions
(In Progress)
Grbin (1996) reported that some wines express mousy taint after oxidation [14], however, ATHP in food has been identified as being very volatile in the presence of oxygen [68][19].
Shilpi Halemane from Logsdon Farmhouse Ales anecdotally reported getting THP in bottles that were not purged properly on their bottling line, and this was detected at the end of the day on the same day [69] (~14:50 mins in).
Lars Meiner reported THP development after heat pasteurizing a batch of homebrew.
https://pubmed.ncbi.nlm.nih.gov/22508009/
THP in Kettle Souring
THP or THP-like flavors have been reported in the kettle souring process in commercial brewing. This could be caused by using a heterofermentative species of Lactobacillus that is able to produce THP, but it has also been reported when an extended second boil was performed. Alex Wright reported that At Halo Brewery brewmaster Callum Hay was able to remove a THP-like flavor from their kettle sours by eliminating the boil time during the pasteurization step of kettle souring (the second boil) and displacing the trub at the bottom of the kettle. It was hypothesized that the boiling and perhaps the trub at the bottom of the kettle that built up after a 24 hour souring process was being heated by their direct fire system, causing Maillard reactions that resulted in pyradines. By scrapping the trub off of the bottom of the boil kettle, and replacing the second boil with a 75°C (167°F) 10 minute rest/whirlpool, they were able to remove this flavor [70]. However, Alan Simons (and possibly others) contradict this by reporting no THP-like flavors when doing an extended second boil after kettle souring [71]. Josh Kauffman reported that by switching from a 60 minute post-boil (after souring the wort with Lactobacillus) to a 15-20 minute pasteurization rest at 180°F reduced the THP-like flavors in their kettle soured beer [72]. Several other brewers in MTF have also reported reducing THP-like flavors by shortening the second boil. There are no controlled experiments that we know of that confirm any of these reports.
Whether or not THP compounds could be created in beer via Maillard reactions has not been proven, and is unlikely. Richard Preiss offered another hypothesis that perhaps the turbulence and heat of boiling inflicts more lysed (destroyed) cells, which release THP into the beer, and that perhaps pasteurization at a lower temperature kills the cells but does not destroy their cell walls [73][74].
Thresholds and Quantities Found in Mousy Wine
- Editor's note: the following thresholds are from studies on wine, and may not hold true for beer. As stated above, detection is influenced by pH, and so the low pH of sour beer may have the similar effect of repressing odor (more so) and taste (less so) detection, whereas non-sour Brettanomyces beers may have a higher detection rate.
- ETHP
- Odor threshold (wine): 150 µg/L
- Concentration reported in wines exhibiting mousy off-flavour: 2.7-18.7 µg/L (ETHP is generally not the cause of the detected mousy off-flavor)
- ATHP
- Odor threshold (water): 1.6 µg/L
- Concentration reported in wines exhibiting mousy off-flavour: 4.8-106 µg/L (ATHP is generally the cause of the detected mousy off-flavor)
- APY
Since a low pH hinders the detection of THP, one detection used by winemakers is to rub some wine on one's palm and smelling for THP. A more reliable method is to dip an alkaline paper strip into the wine, and then smelling the strip to detect the aroma of THP [14].
Off Flavor Kits
Discussions
What We Don't Know
- How does oxygen affect THP production biochemically?
- How much oxygen has an effect, and is there a maximum amount of dissolved oxygen that would be insignificant in its production?
- Does bottle orientation during storage have an effect? (Jeff Porn and Shawn Savuto both reported THP only when storing bottles horizintally; see Bottle Orientation).
- Does creating an acid tolerance starter for conditioning yeast affect THP production?
- How do THP off-flavors age out of beer (hypothesis: ATHP is metabolized into ETHP by Brettanomyces, or somehow chemically degraded into ETHP)?
- Does storage temperature play a role in the degradation of THP off-flavors (cold vs room temp vs hot)?
- What genetic phenotypes determine high THP production in some strains of Brettanomyces and not others?
- What role does acetic acid bacteria play in THP production?
- What are the unidentified transient forms of THP, and do they apply to beer or just wine?
- Does the release of lysine from yeast autolysis when aging on trub increase THP potential (some say it does in wine and cider [77])?
- Are other species of yeast such as wild S. cerevisiae capable of producing THP [78]?
- Do sulphites bind to THP molecules, rendering them unperceivable [79]?
* Due to the specialized GC/MS equipment needed for measuring forms of THP that most labs do not have, certain answers will be difficult to obtain. Some studies have used alkaline strips as a way to smell the aroma of THP, and while not as precise as the specialized GC/MS lab equipment, could still help further the understanding of THP. These strips were prepared in the following way: knowing that mousy off-flavour has a lingering sensory impact, the technique of alkaline paper strip assessment was adapted from Heresztyn (1986a). Paper strips (Whatman No. 1, 4–5 mm × 50 mm) were prepared by soaking in NaOH (0.1M) and drying overnight at room temperature. The alkaline paper strips were then briefly dipped into cell-free samples (centrifuged) and immediately assessed for the mouse-like odour by sniffing [80].
MTF Threads and Other Forum Posts
Below is a list of discussions on internet forum threads that may shed light on specific strains and individual experiences. Keep in mind that many of the opinions and experiences are anecdotal, although commonalities and shared experiences may prove to be useful and accurate.
- MBAA podcast episode 326, "THP" with Paulina Streimikyte and Glen Fox.
- Joe Idnoni from House Cat Brewing and Brian Ogden from Attaboy Beer report that Caseinate de Potassium (a fining agent used in wine-making) was used to significantly reduce THP off-flavors in a kettle sour beer. Note that casein is a milk allergen, and might require declaration as a milk allergen (refer to your local government requirements) [81].
- Tariq Ahmed of Revel Cider discusses their protocol for reducing THP in spontaneously fermented ciders.
- Chris Cates with thoughts on water profile (sulfate) increasing the perception of THP in sour beer.
- MTF thread detailing experiences with certain strains and procedures (03/24/2017).
- Milk The Funk thread on THP flavor spikes and other sensory testing approaches.
- Milk The Funk thread on anecdotal experiences of THP forming in beer lines.
- Milk The Funk thread reporting an anecdote by Henrik Ventzel that adding some DME and fresh Nottingham yeast to a sour with THP and cleared up THP after 2 months.
- General Milk The Funk Thread on March 10, 2015.
- General Milk The Funk Thread on Aug 25, 2015.
- General Milk The Funk Thread on 09/17/2015.
- MTF thread on THP in natural wine, and increased awareness of THP in wine. as a result.
- MTF thread on removing THP from wine made with low or no sulphite.
- MBAA Podcast 237: David Fuhrer on bottle conditioning using the German speise method (similar to krausening) reduces THP.
- Homebrewtalk thread started by "ne0t0ky0".
- Homebrewtalk thread started by "loctones", comments by Michael Tonsmeire.
- Babblebelt thread with comments by Chad Yakobson.
- Michael Tonsmeire observes that adding fresh Saccharomyces at bottling time seems to reduce THP production, versus force carbonating.
- Kyle Weniger from Joseph James Brewing Co noticed significantly improved THP reduction storing a split batch of beer at room temperature versus storing cold.
- Chad Yakobson on The Sour Hour, 3/11/2015 (around 50 minute mark) - indicates that he has been researching THP for 2 years, and is continuing this research. Little else is mentioned.
See Also
Additional Articles on MTF Wiki
External Resources
- THP overview presentation by Richard Preiss at Escarpment Laboratories.
- "The Wine Flaw of Our Times," by John McCarroll.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Moulis, P., Miot-Sertier, C., Cordazzo, L., Claisse, O., Franc, C., Riquier, L., Albertin, W., Marchand, S., De Revel, G., Rauhut, D., & Ballestra, P. (2023). Which microorganisms contribute to mousy off-flavour in our wines?. OENO One, 57(2), 177–187. https://doi.org/10.20870/oeno-one.2023.57.2.7481.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 Mousy Off-Flavor: A Review. Eleanor M. Snowdon, Michael C. Bowyer, Paul R. Grbin, and Paul K. Bowyer. 2006.
- ↑ "6-Acetyl-2,3,4,5-tetrahydropyridine". Wikipedia. Retrieved 07/22/2016.
- ↑ Brettanomyces Bruxellensis, Essential Contributor in Spontaneous Beer Fermentations Providing Novel Opportunities for the Brewing Industry. Jan Steensels. BrewingScience, Sept/Oct 2015 (Vol. 68). 2015.
- ↑ Jancis Robinson. "The Oxford Companion to Wine". Oxford University Press. Sep 17, 2005. Pg 483.
- ↑ MTF thread about how long THP takes to age out with comment by Mark Trent. 10/24/2016.
- ↑ MTF discussion regarding THP degredation under room temperature versus refrigeration temperatures. 10/28/2016.
- ↑ Tonsmeire, Michael. Homebrewtalk.com post 1. 11/21/2014. Retrieved 3/10/2015.
- ↑ Tariq Ahmed and Shane Martin. Milk The Funk thread about THP aging out of cider at room temperature. 01/30/2018.
- ↑ Ryan Sandlin. Milk The Funk Facebook group thread on THP aging out after 90 days of room temperature storage. 04/23/2018.
- ↑ Tonsmeire, Michael. Homebrewtalk.com post 2. 11/21/2014. Retrieved 3/10/2015.
- ↑ MTF discussion on whether or not THP should always be considered an off-flavor. 10/27/2017.
- ↑ Esther Mobley. San Francisco Chronicle. 09/24/2019. Retrieved 09/25/2019.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 Developments in the sensory, chemical and microbiological basis of mousy taint in wine. Grbin, P.R, Costello, P.J, Herderich, M. 1996.
- ↑ 15.0 15.1 15.2 PAUL R. GRBIN and PAUL A. HENSCHKE. 2000.
- ↑ 16.0 16.1 16.2 16.3 16.4 The Role of Lysine Amino Nitrogen in the Biosynthesis of Mousy Off-Flavor Compounds by Dekkera anomala. Paul R. Grbin, Markus Herderich, Andrew Markides, Terry H. Lee, and Paul A. Henschke. J. Agric. Food Chem., 2007.
- ↑ Quantitative analysis of mousy off-flavour compound 2-acetyl tetrahydropyridine in wine using liquid chromatography tandem mass spectrometry interfaced with atmospheric chemical ionisation. Y.Hayasaka. 2018. DOI: https://doi.org/10.1016/j.chroma.2018.12.047.
- ↑ Private correspondence with Dr. Paul Grbin by Dan Pixley. 11/2/2017.
- ↑ 19.0 19.1 Comparison between standardized sensory methods used to evaluate the mousy off-flavor in red wine. Tempère, S., Chatelet, B., de Revel, G., Dufoir, M., Denat, M., Ramonet, P.-Y., Marchand, S., Sadoudi, M., Richard, N., Lucas, P., Miot-Sertier, C., Claisse, O., Riquier, L., Perello, M.-C., & Ballestra, P. 2019. DOI: https://doi.org/10.20870/oeno-one.2019.53.2.2350.
- ↑ Daiki Kiyomichi, Céline Franc, Pierre Moulis, Laurent Riquier, Patricia Ballestra, Stéphanie Marchand, Sophie Tempère, Gilles de Revel. Investigation into mousy off-flavor in wine using gas chromatography-mass spectrometry with stir bar sorptive extraction. Food Chemistry, Volume 411, 2023, 135454, ISSN 0308-8146, https://doi.org/10.1016/j.foodchem.2023.135454.
- ↑ Paulina Martusevice, Xueqi Li, Matt Hengel, Selina C. Wang, Glen Fox, Analysis of mousy off-flavor compound 2-Acetyl-tetrahydropyridine using Liquid Chromatography Mass Spectrometry with Electrospray Ionization in sour beer, MethodsX, Volume 12, 2024, 102643, ISSN 2215-0161, https://doi.org/10.1016/j.mex.2024.102643.
- ↑ Screening for 2-acetyl-1-pyrroline in the headspace of rice using SPME/GC-MS. Grimm CC, Bergman C, Delgado JT, Bryant R. 2001.
- ↑ "testing for mouse". Testing for Mouse. Retrieved 04/06/2021.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 Chemistry of 2-Acetyl-1-pyrroline, 6-Acetyl-1,2,3,4-tetrahydropyridine, 2-Acetyl-2-thiazoline, and 5-Acetyl-2,3-dihydro-4H-thiazine: Extraordinary Maillard Flavor Compounds. An Adams and Norbert De Kimpe. 2005.
- ↑ Primary odorants of pale lager beer. Peter Schieberle. 1991.
- ↑ <Formation of 2-Acetyl-l-pyrroline and Other Important Flavor Compounds in Wheat Bread Crust. Peter Schieberle. 1989. DOI: 10.1021/bk-1989-0409.ch025.
- ↑ Abiotic and Biotic Factors Controlling Grain Aroma along the Value Chain of Fragrant Rice: A Review. Ayut Kongpun, Tonapha Pusadee, Pennapa Jaksomsak, Kawiporn chinachanta, Patcharin Tuiwong, Phukjira Chan-In, Sawika Konsaeng, Wasu Pathom-Aree, Suchila Utasee, Benjamapohn Wangkaew, Chanakan Prom-U-Thai. Rice Science. 2023. https://doi.org/10.1016/j.rsci.2023.11.004.
- ↑ 2-Acetylpyridine. Wikipedia. Retrieved 09/04/2018.
- ↑ 1-pyridin-2-ylethanone. The Good Scents Company website. Retrieved 09/04/2018.
- ↑ 2-acetyl pyridine. Aroxa website. Retrieved 09/04/2018.
- ↑ Basic compounds contributing to beer flavour. Richard J. Harding, Harry E. Nursten, John J. Wren. 1977. DOI: https://doi.org/10.1002/jsfa.2740280218.
- ↑ A Review of N-Heterocycles: Mousy Off-Flavor in Sour Beer. Paulina Martusevice, Xueqi Li, Matt J. Hengel, Selina C. Wang, and Glen P. Fox. Journal of Agricultural and Food Chemistry 2024 72 (14), 7618-7628. DOI: 10.1021/acs.jafc.3c09776.
- ↑ Managing Wine Quality: Oenology and Wine Quality. A Reynolds Elsevier, Sep 30, 2010. Pg 359.
- ↑ The α-aminoadipate pathway for lysine biosynthesis in fungi. Hengyu Xu, Babak Andi, Jinghua Qian, Ann H. West , Paul F. Cook. Sept 2006.
- ↑ Lysine Biosynthesis in Saccharomyces cerevisiae: Mechanism of α-Aminoadipate Reductase (Lys2) Involves Posttranslational Phosphopantetheinylation by Lys5. David E. Ehmann , Amy M. Gehring , and Christopher T. Walsh. 1999.
- ↑ Elucidation of the Role of Nitrogenous Wort Components in Yeast Fermentation. C. Lekkas, G.G. Stewart, A.E. Hill, B. Taidi and J. Hodgson. May 2012.
- ↑ Proteins and amino acids in beers, their contents and relationships with other analytical data. S. Gorinstein, M. Zemsera, F. Vargas-Albores, J-L. Ochoa, O. Paredes-Lopez, Ch. Scheler, J. Salnikow, O. Martin-Belloso, S. Trakhtenberg. 1999.
- ↑ Amino Acid Composition of Six Grains and Winter Wheat Forage. Morey, D.D. 1983.
- ↑ "Oats". DIY Soylent website. Retrieved 02/07/2017.
- ↑ "Barley malt flour". DIY Soylent website. Retrieved 02/07/2017.
- ↑ "Wheat flour, whole-grain". DIY Soylent website. Retrieved 02/07/2017.
- ↑ Changes in the Amino Acid Composition of the Different Nitrogenous Fractions during the Aging of Wine with Yeasts. Victoria Moreno-Arribas, Encarnación Pueyo, M. Carmen Polo, and Pedro J. Martín-Álvarez. 1998. DOI: 10.1021/jf9803381.
- ↑ Influence of the yeast strain on the changes of the amino acids, peptides and proteins during sparkling wine production by the traditional method. Martínez-Rodríguez AJ, Carrascosa AV, Martín-Alvarez PJ, Moreno-Arribas V, Polo MC. 2002. DOI: 10.1038/sj.jim.7000323.
- ↑ New trends on yeast autolysis and wine ageing on lees: a bibliographic review. Caroline Fornairon-Bonnefond, Carole Camarasa, Michel Moutounet, Jean-Michel Salmon. 2002.
- ↑ Richard Preiss. Milk The Funk Facebook group thread on on yeast autolysis impact on THP. 04/10/2018.
- ↑ Yakobson, Chad. The Brettanomyces Project; Introduction. Retrieved 3/10/2015.
- ↑ 47.0 47.1 Significance of Brettanomyces and Dekkera during Winemaking: A Synoptic Review. A. Oelofse, I.S. Pretorius, and M. du Toit. 2008.
- ↑ How the Purity of Sparged Carbon Dioxide Affects the Oxygen Concentration of Beer. Tap Into Hach blog. 01/24/2014. Retrieved 06/29/2017.
- ↑ How the Purity of Injected Carbon Dioxide Affects the Oxygen Concentration of Beer. Tap Into Hach blog. 12/02/2013. Retrieved 06/29/2017.
- ↑ Mitch Ermatinger. Milk The Funk Facebook group post on THP reduction using CBC1 and EC1118. 10/03/2017.
- ↑ Justin Amaral and Mike Karnowski on purging bottles with CO2. Milk The Funk Facebook group. 12/13/2017.
- ↑ Growth and volatile compound production by Brettanomyces/Dekkera bruxellensis in red wine. Romano A, Perello MC, de Revel G, Lonvaud-Funel A. J Appl Microbiol. 2008 Jun.
- ↑ Joseph, C.M. Lucy. Aromatic Diversity of Brettanomyces. U.C. Davis. Retrieved 3/10/2015.
- ↑ 54.0 54.1 54.2 54.3 Moulis, P., Miot-Sertier, C., Franc, C., Riquier, L., Beisert, B., Marchand, S., … Ballestra, P. (2024). Impact of Pediococcus parvulus and Saccharomyces cerevisiae on Brettanomyces bruxellensis mousy compound production. OENO One, 58(3). https://doi.org/10.20870/oeno-one.2024.58.3.8060
- ↑ 55.0 55.1 Ability of lactic acid bacteria to produce N-heterocycles causing mousy off-flavour in wine. PETER J. COSTELLO1, TERRY H. LEE1, and PAULA. HENSCHKE. 2008.
- ↑ 56.0 56.1 56.2 56.3 Mousy Off-Flavor of Wine: Precursors and Biosynthesis of the Causative N-Heterocycles 2-Ethyltetrahydropyridine, 2-Acetyltetrahydropyridine, and 2-Acetyl-1-pyrroline by Lactobacillus hilgardii DSM 20176. Peter J. Costello and Paul A. Henschke. 2002.
- ↑ Formation of Substituted Tetrahydropyridines by Species of Brettanomyces and Lactobacillus Isolated from Mousy Wines. Tamila Heresztyn. 1986.
- ↑ Sparrows, Jeff. Wild Brews. Brewers Publications. 2005. Pg. 112.
- ↑ Lahtinen, Ouwehand, Salminen, von Wright. Lactic Acid Bacteria: Microbiological and Functional Aspects, Fourth Edition. Pg 348.
- ↑ Heresztyn, Tamila. Formation of Substituted Tetrahydropyridines by Species of Brettanomyces and Lactobacillus Isolated from Mousy Wines.
- ↑ UniProt article. Retrieved 3/10/2015.
- ↑ UniProt article. Retrieved 3/10/2015.
- ↑ UniProt article. Retrieved 3/10/2015.
- ↑ [https://ives-technicalreviews.eu/article/view/9206 "Does Saccharomyces cerevisiae play a supporting role in mousy off-flavours production?" Pierre Moulis, Cécile Miot-Sertier, Céline Franc, Laurent Riquier, Beata Beisert, Stéphanie Marchand, Gilles de Revel, Doris Rauhut, Patricia Ballestra. Published: 7 March 2025. DOI: https://doi.org/10.20870/IVES-TR.2025.9206.]
- ↑ The role of free amino acids present in yeast as precursors of the odorants 2-acetyl-1-pyrroline and 2-acetyltetrahydropyridine in wheat bread crust. Peter Schieberle. 1990.
- ↑ Xiatao Zhou, Liqiong Wen, Jinshan Xiao, Xueying Mo, Peng Wan, De-Wei Chen. 2- Acetyl-1-pyrroline originated from Maillard reaction is the key odorant of cooked Lipu taro. International Journal of Gastronomy and Food Science, 2024, 100968. ISSN 1878-450X. https://doi.org/10.1016/j.ijgfs.2024.100968.
- ↑ 2-Oxopropanal, Hydroxy-2-propanone, and 1-PyrrolineImportant Intermediates in the Generation of the Roast-Smelling Food Flavor Compounds 2-Acetyl-1-pyrroline and 2-Acetyltetrahydropyridine. Thomas Hofmann, and Peter Schieberle. 1998. DOI: 10.1021/jf970990g.
- ↑ Reactivity and stability of selected flavor compounds. Monthana Weerawatanakorn, Jia-Ching Wu, Min-Hsiung Pan, Chi-Tang Ho. 2015. DOI: https://doi.org/10.1016/j.jfda.2015.02.001.
- ↑ "Wild Beer Curling: Course Correcting and Guiding Your Beer to Success". HomebrewCon Seminar. 2018.
- ↑ Alex Wright. Milk The Funk Facebook group thread on THP in kettle sours. 11/24/2018.
- ↑ Alan Simons. Milk The Funk Facebook group thread on THP in kettle sours. 11/24/2018.
- ↑ Josh Kauffman. Milk The Funk Facebook group thread about THP in kettle sours. 12/18/2018.
- ↑ Kristen England and Matt Humbard. Milk The Funk Facebook group thread on THP compounds being formed in kettle sours via Maillard reactions. 12/28/2018.
- ↑ Richard Preiss. Milk The Funk Facebook group thread on THP in kettle sours. 01/03/2019.
- ↑ Malolactic Fermentation 2005. Geneva on the Lake. Feb 2005. Retrieved 3/10/2015.
- ↑ Richard Preiss. Milk The Funk Facebook group thread on THP spikes. 01/03/2018.
- ↑ Tariq Ahmed of Revel Cider. Milk The Funk Facebook thread on THP in wines/ciders aged on lees. 10/16/2017.
- ↑ Zach Taggart. Milk The Funk Facebook group thread on other yeast species producing THP. 04/24/2018.
- ↑ Otto Forsberg. Milk The Funk Post discussion on THP and sulfites. 04/27/2018.
- ↑ Private correspondence with Dr. Paul Grbin by Dan Pixley. 11/2/2017.
- ↑ "UPDATED - Allergy Alert on Undeclared Milk in Nutrition Resource Services, Inc.'s Whey, Casein, and Colostrum Protein Products". FDA website. 08/03/2015. Retrieved 12/21/2017.
