Difference between revisions of "Laboratory Techniques"
m (→Saccharomyces) |
|||
| Line 49: | Line 49: | ||
===''Saccharomyces''=== | ===''Saccharomyces''=== | ||
| − | A wide variety of media | + | A wide variety of media can be used for ''Saccharomyces''. Bromocresol Green can also be added to these media, as in the commercial WLN formulation. Most ''Saccharomyces'' cannot metabolize this dye, causing the colonies to stain green. Chloramphenicol can also be added to eliminate bacterial growth. Although not all of these media are specifically for your average brewers ''Saccharomyces'', most strains should have no issues growing. |
'''YPD Media''' | '''YPD Media''' | ||
Revision as of 18:49, 6 September 2017
This page will focus on home lab and small brewery lab techniques.
Contents
Equipment
(To do)
Dissolved Oxygen Meter
Density Meter
Anton Paar Portable Density Meter
Titratable Acidity Meter
UV/VIS Spectrophotometer
Growth Media
Lactobacillus/Pediococcus
See Rogosa SL Agar
MRS Media
| Chemical | Usage Amount |
|---|---|
| Dextrose | 20 grams |
| Peptone | 10 grams |
| Beef Extract | 8 grams |
| Yeast Extract | 4 grams |
| Sodium Acetate | 5 grams |
| Dipotassium Hydrogen Phosphate | 2 grams |
| Ammonium Citrate | 2 grams |
| Manganous Sulfate Tetrahydrate | 0.05 grams |
| Magnesium Sulfate Heptahydrate | 0.2 grams |
| Distilled/De-ionized Water | Fill to 1000 ML |
Saccharomyces
A wide variety of media can be used for Saccharomyces. Bromocresol Green can also be added to these media, as in the commercial WLN formulation. Most Saccharomyces cannot metabolize this dye, causing the colonies to stain green. Chloramphenicol can also be added to eliminate bacterial growth. Although not all of these media are specifically for your average brewers Saccharomyces, most strains should have no issues growing.
YPD Media
| Chemical | Usage Amount |
|---|---|
| Yeast Extract | 10 grams |
| Peptone | 20 grams |
| Dextrose | 20 grams |
| Agar(optional) | 15 grams |
| Distilled Water | Fill to 1000 ML |
MYPG Media
| Chemical | Usage Amount |
|---|---|
| Malt Extract | 3 grams |
| Yeast Extract | 3 grams |
| Peptone | 3 grams |
| Dextrose | 10 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 ML |
Sabouraud Media
| Chemical | Usage Amount |
|---|---|
| Cycloheximide (Optional) | 10 mg |
| Chloramphenicol (Optional) | 0.5 grams |
| Peptone | 5 grams |
| Dextrose | 20 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 ML |
Freezing Media
| Chemical | Usage Amount |
|---|---|
| Glycerin | 50 grams |
| Ascorbic Acid | 15 grams |
| Liquid YPD/MYPG | Fill to 100 ML |
Beef Broth Media
| Chemical | Usage Amount |
|---|---|
| Beef Broth(No preservatives) | 500 mL |
| NaCI (can substitute non-iodized or sea salt) | 50-200 grams |
| Peptone | 5 grams |
| Dextrose | 10 grams |
| Agar(optional) | 17 grams |
| Distilled Water | Fill to 1000 ML |
Wild Yeast Screening Media
| Chemical | Usage Amount |
|---|---|
| Peptone | 5 grams |
| Yeast Extract | 3 grams |
| Malt Extract | 3 grams |
| Dextrose | 5 grams |
| CuSO4 | 310 mg |
| Distilled Water | Fill to 1000 ML |
Brettanomyces
A few different medias can be used to isolate Brettanomyces but DBDM media is commonly used. WLD with additions of cycloheximide can also be used.
DBDM Media Recipe
| Chemical | Usage Amount |
|---|---|
| Yeast nitrogenous base (YNB) | 6.5 grams |
| Ethanol | 6% v/v |
| Cycloheximide | 10 mg |
| p-coumaric acid | 100 mg |
| Bromocresol Green | 22 mg |
| Agar | 20 grams |
| Distilled Water | Fill to 1000 ML |
WLD Media Recipe
| Chemical | Usage Amount |
|---|---|
| Cycloheximide | 4 grams |
| Yeast Extract | 4 grams |
| Pancreatic Digest of Casein(Pepton) | 5 grams |
| Dextrose | 50 grams |
| Monopotassium Phosphate | .55 grams |
| Potassium Chloride | 425 mg |
| Calcium Chloride | 125 mg |
| Magnesium Sulfate | 125 mg |
| Ferric Chloride | 2.5 mg |
| Manganese Sulfate | 2.5 mg |
| Bromocresol Green | 22 mg |
| Agar | 20 grams |
| Distilled Water | Fill to 1000 ML |
Misc/Other
Reference on DBDM: https://www.facebook.com/groups/MilkTheFunk/permalink/1805210829507123/?comment_id=1805397166155156&comment_tracking=%7B%22tn%22%3A%22R%22%7D
Storage
Propagators
Justin Amaral's 4.5 BBL Propagator
The propagator below has a 14 gallon capacity, allowing a prop up of 4.5 BBL. This same set up can be used on any fermenter but is bested used on ones that can hold 5 PSI and up. The seal on the below fermenter can hold around 5 PSI but clamps are also used on the lid to ensure it can hold up to 10 PSI. It essentially acts as a large stir plate while allowing to trickle in either O2, NO2 or CO2 depending on what your propagating. Ideally you'd also use a non magnetic drive pump but its not essential.
Below is the draw up of the build out for this prop up tank. As you can see it uses quick disconnects but you can use tri-clover connects as well. It also uses a diffusion stone that connects into one of the ferrules allowing you to trickle in gases. The picture shows a diffusion stone directly into the fermenter but its since been adapted as shown in the other pictures below to allow the diffusion stone to be behind a valve so its only exposed when needed. It also uses a whirlpool connector that goes into the fermenter creating the whirlpool when the pump is on.
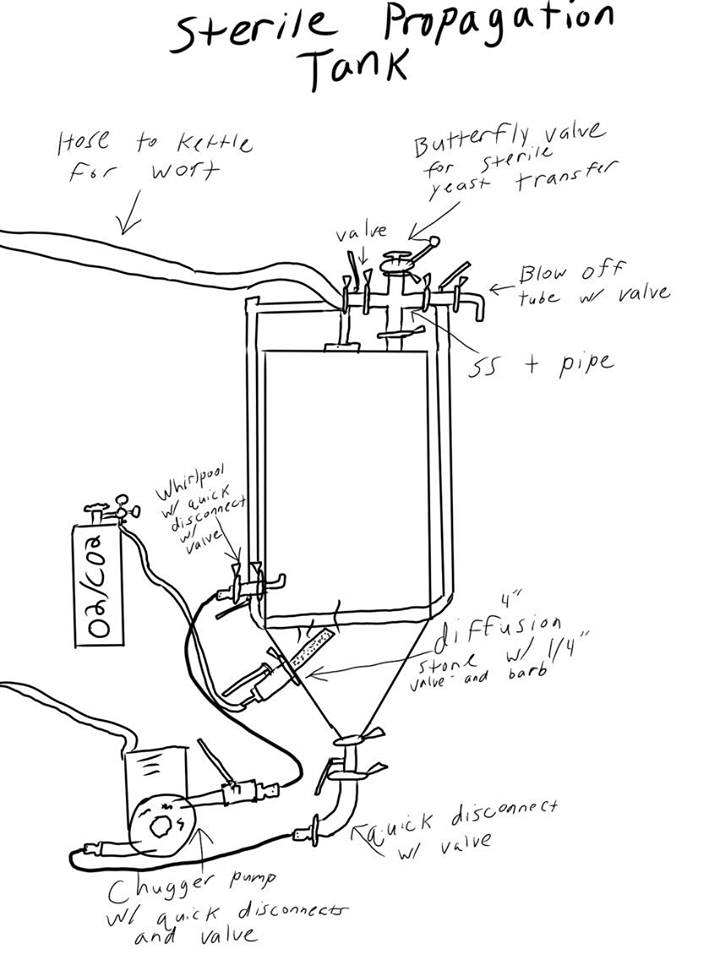
Below you can see the 4 way SS T valve at the top of the fermenter allowing for a blow off tube, sterile transferring of yeast(the yeast has to be in a container with a tri-clover valve for this), and the hose coming from the kettle.

Below is the valve set up. The top valve has the whirlpool connect, the middle has the diffusion stone behind the valve, and the bottom has an elbow with a valve.
Techniques
(Videos provided by Bryan of Sui Generis blog and Zach Taggart.)
Aseptic Technique
Making Agar Plates
- Making WLD plates:
Yeast/Bacteria Isolation
- Mark Trent details his method for isolating Brettanomyces from Saccharomyces from dregs or other mixed cultures.
- See Wild Yeast Isolation.
- See isolating Lactobacillus.
Making Your Own Medias
Although media's can be bought pre-made, you can also make these media's yourself. Media's can be either sterilized via an autoclave/pressure cooker or using sterile filtering. Keep in mind it can be difficult however to sterile filter some items such as yeast extract, peptone and brewer's grade DME. Because of this some just autoclave parts of the media while sterile filtering the rest into the sterilized media.
It is crucial you use aspect technique with your media once it is sterilized to prevent any contamination. If you are storing extra media for later use make sure to remake it every month or 2 if unused.
Yeast Banking
Cell Counting
Gram Staining
Shipping Cultures
DeWayne Schaaf recommends the following procedures for shipping cultures with the USA states and territories [1]:
- Use 15ml centrifuge tubes from Cynmar LLC - Wine & Brew. They are great quality and have a very low rate of leakage, especially when combined with electrical tape.
- Place each liquid filled tube into its own snack sized baggie to minimize cross contamination if they do happen to leak.
- Each of these smaller bags will be placed into a quart Ziplock.
- My main shipping container are Uline Poly bubble mailers. I don't tend to use ice packs as I've found them unnecessary when shipping in favorable temps (use ice packs during the summer).
- USPS is my preferred shipping method. Using first class shipping, my packages typically take no more than 3 days to reach somewhere in the USA and cost right around $3, and that includes a tracking number. Shipping rates are roughly the same for any USA state or territory. The 48 continental states, Alaska, Hawaii, Puerto Rico, and Guam all cost roughly the same.
For 5 gallon pitches or cultures that may still be a bit active creating cO2 Soda Preform tubes work very well. These are the same type of tubes White Labs uses for their homebrew pitches. https://www.amazon.com/Soda-Bottle-Preforms-Caps-30/dp/B008MB1QNY/ref=sr_1_1?ie=UTF8&qid=1504524577&sr=8-1&keywords=soda+test+tubes
See also:
See Also
Additional Articles on MTF Wiki
- Microscope
- PH Meter
- Wild Yeast Isolation
- Titratable Acidity
- Saccharomyces
- Brettanomyces
- Pediococcus
- Lactobacillus

