Difference between revisions of "Brettanomyces"
(updated all occurences of "Brett" to "Brettanomyces") |
(→Storing Brett) |
||
| Line 332: | Line 332: | ||
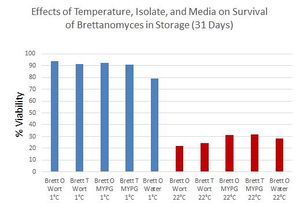
[[File:Brett storage MarkTrent.jpg|thumb|300px|[https://www.facebook.com/groups/MilkTheFunk/permalink/1115768398451373/?comment_id=1145139948847551&offset=0&total_comments=69&comment_tracking=%7B%22tn%22%3A%22R9%22%7D Mark Trent repeated Richard Preiss's results of storing Brett at different temperatures, and also in different mediums.]]] | [[File:Brett storage MarkTrent.jpg|thumb|300px|[https://www.facebook.com/groups/MilkTheFunk/permalink/1115768398451373/?comment_id=1145139948847551&offset=0&total_comments=69&comment_tracking=%7B%22tn%22%3A%22R9%22%7D Mark Trent repeated Richard Preiss's results of storing Brett at different temperatures, and also in different mediums.]]] | ||
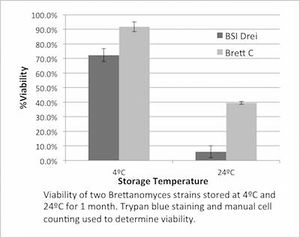
| − | Yakobson's observations were not scientifically quantified and details of his process are lacking (how was the ''Brettanomyces'' stored?), as far as we know. Richard Preiss of [http://www.escarpmentlabs.com/ Escarpment | + | Yakobson's observations were not scientifically quantified and details of his process are lacking (how was the ''Brettanomyces'' stored?), as far as we know. Richard Preiss of [http://www.escarpmentlabs.com/ Escarpment Labs] shared the results of a controlled experiment on MTF that showed that BSI's ''Brett brux Drie'' and WLP645 ''B. claussenii'' survived better in low ABV beer when stored at refrigeration temperatures rather than room temperatures, contradicting the anecdotal observations reported by Yakobson. The samples were grown in 1.040 DME wort until typical cell density was reached, and measured for >95% viability after growth with trypan blue stain and microscopy. 10ml samples of each were stored in sterile conical tubes for one month at different temperatures (4°C and 24°C). The samples were burped to avoid having head pressure as a variable. Trypan blue stain and microscopy were used to measure the viability after one month. After one month of storage at 4°C (39.2°F), the viability of ''B. claussenii'' was 92%, and BSI Drie was 72% viability. The samples stored for one month at 24°C (75.2°F) showed a significant drop in viability, with ''B. claussenii'' ending up at 40% and BSI Drie at 6% viability. This experiment also indicates that the viability of ''Brettanomyces'' strains/species after storage is strain/species dependent <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1115768398451373/ Richard Preiss Brett storage experiment results on MTF. 7/24/2015.]</ref>. |
| − | Mark Trent repeated Richard | + | Mark Trent repeated Richard Preiss's experiment, but tested different mediums (wort, liquid MYPG, and water). Two ''Brettanomyces'' isolates, one from Orval and one from SARA Bernice, were grown in 10 degree Plato wort and MYPG. The isolates are labeled on the chart as "Brett O (Orval)" and "Brett T (Tim Clifford)" respectively. After growth was complete, 10 mL of aliquots were aseptically transferred to 15 mL centrifuge tubes. In addition, the Orval isolate was grown on a MYPG plate and 3 single colonies for each treatment were transferred to 1 mL of sterile RO water in a 2 mL glass tube. Each treatment was prepared in a duplicate and stored at either 22°C or 1°C. Viability was measured after 31 days. Data shown in the chart to the right. No other statistics were performed (there were no statistically significant differences between the different types of storage mediums at room temperature). All storage mediums shared results similar to Richard's results. This further shows that ''Brettanomyces'' survival is a function of temperature, with lower temperatures being beneficial towards survival <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1115768398451373/?comment_id=1145139948847551&offset=0&total_comments=69&comment_tracking=%7B%22tn%22%3A%22R9%22%7D Conversation with Mark Trent on MTF. 09/10/2015.]</ref>. |
Questions raised by MTF members in regards to these results: | Questions raised by MTF members in regards to these results: | ||
Revision as of 22:31, 28 November 2015
Brettanomyces, also referred to by brewers as "Brett" or "Bretta", is a yeast that was originally thought of as a spoilage yeast. The genus name Dekkera is used interchangeably with Brettanomyces, as it describes the teleomorph or spore forming form of the yeast [1]. Known for it's barnyard, fecal, horsey, metallic or Band-Aid flavors, Brettanomyces was unwelcome in most breweries. However, in some styles like Saison and Lambic these flavors add a layer of complexity to the beer. Brettanomyces can form a pellicle. See Lactobacillus, Pediococcus, Saccharomyces, and Mixed Cultures charts for other commercially available cultures.
Contents
Introduction of Characteristics
Brettanomyces, first discovered by Hjelte Claussen in 1904, has traditionally been identified as a contaminate in wineries and brewers due to some of the phenols that it produces. These have generally been described as barnyard, burnt plastic, wet animal, fecal, and horse sweat [2]. However, positive flavor components have been identified in beer such as pineapple, stone fruits, and to some degree acetic acid.
Brettanomyces has been identified on the skins of fruit [2], as well as vectors (insects) [3]. Brettanomyces is not considered to be airborne, however studies have found a very small amount of cells in the air at wineries where wine with Brettanomyces in it was being handled (most of the yeasts found in the air were Aureobasidium and Cryptococcus, which aren't considered spoilage organisms in beer and wine). These set of studies also determined that very specific methodology was needed in order capture Brett from the air, and indicated that the yeast was "stressed". [4]. While it is possible for Brettanomyces to be briefly carried by gusts of air, it only happens in the vicinity where the Brettanomyces is actively fermenting. Good cleaning and sanitation, and cold temperatures should be employed to keep Brettanomyces from infecting other equipment, and flying insects are a more likely cause for cross contamination of Brettanomyces.
It is common in scientific literature to see the names Dekkera and Brettanomyces used as the genus name, with Dekkera being the teleomorph version and Brettanomyces being the anamorph. There are five species within the genus of Brettanomyces: B. anomola, B. bruxellensis, B. custersianus, B. nanus, and B. naardenensis. B. anomola and B. bruxellensis are the only two species that have been identified to have a teleomorph version; in their teleomorph version they are referred to as Dekkera anomola and Dekkera bruxellensis [2]. All of the other names such as the ones often used by yeast labs are derived by old nomenclature that is no longer used. The addition of the addition of small amounts of O2 stimulates glucose fermentation, as well as H+ acceptors such as acetaldehyde, acetone, pyruvic acid and other carbonyl compounds [5].
Unlike most genres of yeast, Brettanomyces has the characteristics of being very tolerant to high amounts of alcohol, a pH as low as 2 [6], and low nitrogen sources [2]. Perhaps the most differentiating characteristic of Brettanomyces is its preference to ferment glucose in the presence of oxygen, which is the opposite preference in Saccharomyces. This was initially dubbed the "negative Pasteur effect" by Custers, and later the "Custers effect" [5].
Sulfite and SO2 inhibits the growth of Brettanomyces [7].
Brettanomyces Metabolism
Carbohydrate Metabolism
Brettanomyces strains may possess both alpha and beta glucosidases. These enzymes allow Brettanomyces strains to break down longer chain carbohydrate molecules (including maltose, sucrose, starch, and cellulose) and to liberate glycosidically bound sugars which are unfermentable to Saccharomyces yeasts [8].
Glycosides are sugar molecules connected to other organic compounds such as acids, alcohols, and aldehydes which are flavor and aroma inactive due to the sugar molecule attached. By cleaving off the sugar molecule through glucosidase activity, Brettanomyces species can liberate these compounds (called aglycones) into their aroma-active and flavor-active states, or states that may become flavor and aroma active through further modification[9]. Therefore Brettanomyces strains are able to produce novel flavors and aromas from hops, fruits, and fruit pits that Saccharomyces yeasts cannot produce. In addition, the liberated aroma and flavor active compounds may be further processed by Brettanomyces through ester production or destruction pathways.
Secondary Metabolites
Secondary metabolites are compounds that are not essential to the life of an organism [10]. Brettanomyces will use a range of secondary metabolites to produce many of the fruity and funky esters, phenols, and acids that this genus of yeast has become known for.
Ester Production
Brettanomyces is capable of forming several ethyl esters (derived from ethanol and fatty acids). Among these are ethyl acetate, ethyl lactate and phenethyl acetate, along with the hydrolysis of isoamyl acetate. During non-mixed fermentations where lactic acid and acetic acid are minimal to none, these esters are produced in smaller quantities [11].
| Ester | Precursors | Flavor/Odor Threshold | Molecular Formula | Notes |
|---|---|---|---|---|
| Ethyl acetate (fruity, pineapple, solventy) | Acetic Acid and ethanol | 33ppm (odor), 100ppm (flavor) | C4H8O2 [12] | High flavor threshold; pineapple or pear-like in low amounts and nail polish in high amounts. Increases production with higher temperatures and oxygen. |
| Ethyl butyrate (pineapple, mango, tropical fruit [13]) | Butyric Acid and ethanol | 0.4ppm (flavor) [14] | C6H12O2 [15] | Low levels of production by some species of Brettanomyces; production decreases with higher acidity [16]. Also known as ethyl butanoate [15]. |
| Ethyl caproate (sweet, fruity, pineapple, banana, apple or aniseed) | Caproic acid and ethanol [17] | 0.2ppm (flavor) [18] | C8H16O2 [19] | Also known as Ethyl hexanoate, Ethyl butyl acetate, and butylacetate [20] |
| Ethyl caprylate (Sweet, waxy, fruity and pineapple with creamy, fatty, mushroom and cognac notes [21]) | Caprylic acid (contained in buckwheat; produced by yeast autolysis) and ethanol [22] | 15ppb (flavor) [23] | C10H20O2 [24] | Also known as ethyl octanoate [24]. |
| Ethyl Decanoate (brandy, fruity, oily, grape) | Capric Acid (Decanoic acid) and ethanol [25] | 0.5ppm (flavor in water) [26] | C12H24O2 [25] | Also known as Ethyl caprate, Ethyl caprinate, and Capric acid ethyl ester [27] |
| Ethyl isovalerate (fruity, sweet, berry-like with a ripe, pulpy fruit nuance [28]) [29][30] | Isovaleric Acid and ethanol | 30ppm (flavor) [28] | C7H14O2 (same as ethyl valerate) [28] | Also found in pineapple, orange juice and peel oil, bilberry, blueberry, strawberry, Swiss cheese, other cheeses, cognac, rum, whiskey, sherry, grape wines, cocoa, passion fruit, mango, and mussels [28]. Also known as Ethyl 3-methylbutanoate [29]. |
| Ethyl lactate (fruity, creamy, rum [31][32]) | Lactic Acid and ethanol | 0.2 ppm-1.66 ppm (odor) [33] | C5H10O3 [34] | Increases production with higher amounts of Lactic Acid [35] |
| Ethyl valerate (Sweet, fruity, acidic, pineapple, apple, green, berry and tropical [36]) [29][30] | Valeric Acid (pentanoic acid) and ethanol | 1500-5000 ppm (odor) [37] | C7H14O2 [36] | Valeric acid quantities found in beer are minimal (0-1 ppm) and below odor threshold [38][39], and is probably also the case for Ethyl valerate. Ethyl valerate is also known as ethyl pentanoate [36]. Also found in apples, bananas, guava, stawberry, cheeses, rum, whiskey, cider, sherry, grape wines, cocoa, coffee, honey, and passion fruit [37]. |
| Isoamyl acetate (banana) | Acetic Acid and Isoamyl alcohol | 1.1ppm (flavor) [40] | C7H14O2 [41] | Produced by certain Saccharomyces strains but concentrations are generally reduced by Brettanomyces. Brettanomyces produces only very small amounts itself [42] |
| Phenethyl acetate (sweet, honey, rose flower like) | Acetyl-CoA and 2-phenylethanol [43][44] | 3-5ppm (odor), 5-10ppm (flavor) [45] | C10H12O2 | Produced in very small amounts in Lambic [42][46]. |
Phenol Production
Phenols such as 4-vinylphenol (4VP, barnyard, medicinal) and 4-vinylguaiacol (4-VG, clove) can be produced in beer by the decarboxylation of hydoxycinnamic acids, which are found in malt. While both Saccharomyces and Brettanomyces strains are have varying capabilities based on strain of converting hydroxycinnamic acids to their vinyl derivatives [47], Brettanomyces is also able to reduce these vinyl derivatives to ethyl derivatives. These vinyl derivatives have similar tastes to the ethyl derivatives but have lower flavor thresholds. Levels of all compounds produced vary depending on species and strain of Brettanomyces.
| Phenol | Phenol Type | Precursors | Flavor/Odor Threshold | Molecular Structure | Notes |
|---|---|---|---|---|---|
| 4-Vinylphenol [48] [49] (Barnyard, Medicinal, Band-aid, Plastic) | Vinyl phenol | p-Coumaric Acid | 0.2 ppm (flavor; in beer) [50] | C8H8O [51] | Production level is different across species/strains of Brettanomyces [52]. |
| 4-Vinylguaiacol [48][49] (Clove) | Vinyl phenol | Ferulic Acid | 0.3 ppm (flavor; in beer) [53] | Also known as 2-methoxy-4-vinyl phenol [54] | C9H10O2 [54]. Produced by some strains of S. cerevisiae [55]. Some Brettanomyces species/strains may also be able to produce this compound at varying levels [29][56][52]. |
| 4-Vinylcatechol [48][49] (Plastic, Bitter, Smokey) | Vinyl phenol | Caffeic Acid | C8H8O2 [57] | Production level is difference across species/strains of Brettanomyces [52]. | |
| 4-Ethylphenol [48][49] (Barnyard, Spicy, Smoky, Medicinal, Band-Aid [58]) | Ethyl phenol | 4-vinylphenol | 0.3 ppm (odor; in beer) [59] | C8H10O [60] | Also known as 1-Ethyl-4-hydroxybenzene and P-Ethylphenol [60]. |
| 4-Ethylguaiacol [48][49] (Smokey, Spicy, Clove [61]) | Ethyl phenol | 4-vinylguaiacol | 0.13 ppm (odor; in beer) [59] | C9H12O2 [62] | Also known as 4-Ethyl-2-methoxyphenol [62]. |
| 4-Ethylcatechol [48][49] (Band‐aide, Medicinal, Barnyard) | Ethyl phenol | 4-Vinylcatechol | C8H10O2 [63] | Also known as 4-ethylbenzene-1,2-diol [63]. |
Acid Production
In the presence of oxygen, Brettanomyces strains are capable of producing acetic acid. Depending on the brewer's palate and the degree of acetic production, this can be a desirable or undesirable trait. The degree of acetic acid production varies among different Brettanomyces strains. Acetic acid produced by Brettanomyces may also be used in the synthesis of acetate esters such as ethyl acetate. Brettanomyces has been shown to produce enough fatty acids in anaerobic fermentation to drop the pH to 4.0, which can also be esterified (see the ester table above) [16]. Many of these acids can have an unpleasant rancid odor and/or taste, which may be noticeable in young Brettanomyces beers before these acids are esterified.
| Acids Produced | Precursors | Notes |
|---|---|---|
| Acetic Acid (Vinegar, hard boiled egg) | Oxygen | Increased production with higher levels of oxygen exposure [16]. |
| Isovaleric Acid (Feety, parmesian) [64][65] | Leucine | Commonly described as a "spoilage" acid produced by Brettanomyces in wine, but also appears in beer. |
| Caproic acid (Fatty, cheesy, waxy, barnyardy) [16] | Fatty acid. | |
| Enanthic acid (Rancid odor) [16] | Fatty acid. | |
| Caprylic acid (Rancid-like smell and taste [16] | Fatty acid. Also found in milk. Gives a waxy/oily mouthfeel. Flavor is more intense at low pH levels. Also called octanoic acid.[66] | |
| Pelargonic acid (Rancid odor) [16] | Fatty acid. | |
| Capric acid (Barnyard animal odor/taste) [16] | Fatty acid. Also found in milk, coconut oil, and seed oils [67]. | |
| Undecylic acid [16] | Fatty acid. | |
| Lauric acid (faint odor of bay oil or soap) [16] | Fatty acid. |
Other Compounds
| Compound Produced | Precursors | Threshold | Notes |
|---|---|---|---|
| Tetrahydropyridine (cheerios, mousy, urine, cracker biscuit) | L-Lysine, ethanol, and oxygen | See the Tetrahydropyridine page for more details. Classified as a ketone [68]. |
Commercial Cultures
Pure cultures. In cooperation with Funk Factory.
Bootleg Biology
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Unknown | Unknown | Unknown | BB0034A – Funk Weapon Series: #1 | This rare, and commercially unavailable yeast isolate, produces pungent horse blanket and fresh leather aromas. Perfect for breaking out the funk in farmhouse-style beers. This is the first release in the Dusty Bottoms Collection’s ongoing Funk Weapon Series of unique, rare Brettanomyces and Brett-like wild yeast cultures. | West Flanders, Belgium brewery specializing in funky, sour, mixed-fermentation beers. |
Brewing Science Institute
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis | Brettanomyces bruxellensis | Medium intensity Brett character. Classic strain used in secondary fermentation for Belgian style beers and lambics. | Same as White Labs. Pro-Brewers only. |
| Claussenii | Dekkera anomala | B. claussenii | Brettanomyces clausenii | Low intensity Brett character. | Same as White Labs. Pro-Brewers only. |
| CMY1 | Dekkera bruxellensis | B. bruxellensis CMY1 | bsi | Chad Yakobson's mutation of BSI Drie. Pro-Brewers only. | |
| Drie | Dekkera bruxellensis | B. drei | Brettanomyces bruxellensis var. Drei | Highly aromatic brett strain. Sourness takes extensive aging to produce | Isolate from Drie Fonteinen; Pro-Brewers only. |
| Lambicus | Dekkera bruxellensis | B. lambicus | Brettanomyces lambicus | High intensity Brett character. Know to produce the “horsey” aroma characteristic of Brettanomyces yeast. Classic strain used in secondary fermentation for Belgian style beers and lambics. Same as White Labs. | Pro-Brewers only. |
East Coast Yeast
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Anomala | Dekkera anomala | B. intermedius | ECY-04 | strong ester profile with some light funk and acidity | beer - Adelaide, Australia |
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis | ECY-05 | funky with barnyard notes accompanied by some fruit | isolated from Belgian stout |
| Custersianus | Dekkera custersiana | B. custersianus | ECY-19 | light fruit and hay | Bantu beer brewery, South Africa |
| Farmhouse | ? | B. fantome | ECY-03b | Fruity and funky profile with some acidity gradually increasing over time. Aeration has more of a muted effect | Isolate from Fantome. May not be Brett as per Lance Shaner on MTF. |
| Naardenensis | Dekkera naardenensis | B. naardenensis | ECY-30 | strawberry, honey, ripe fruit with a tart, citrusy acidity after 6mo of aging | Isolated from Dr. Pepper |
| Nanus | Eeniella nana | B. nanus | ECY-24 | spicy, saison-like profile | bottled beer - Kalmar, Sweden |
Inland Island Brewing & Consulting
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Bruxellensis | Dekkera bruxellensis | B. brux. I | INISBC-901 | Isolated from a brewery in Brussels this particular Brettanomyces strain in known for producing aromatics reminiscent of horse, barnyard, sweat, and goat. It is highly attenuative and will take up to 6 months to fully finish fermentation. It is suggested that this strain be used with another primary fermentation strain. 90% + Attenuation. Low Flocculation. 60-75 F Temperature Range. High Alcohol Tolerance. | Brewery in Brussels |
| Bruxellensis | Dekkera bruxellensis | B. brux. III | INISBC-903 | Isolated from a small brewery just outside of Brussels. Produces an aromatic profile that is more mild than INISBC-901 with increased tropical fruitiness. Able to ferment a beer without added Saccharomyces c. Mixed with lactobacillus this strain will create a wonderful sour beer. 90% + Attenuation. Low Flocculation. 60-75 F Temperature Range. High Alcohol Tolerance. | Small Brewery just outside of Brussels |
| Brett. Barrel Yeast III | INISBC-913 | Strain is able to ferment without added help from another Saccharomyces c. strain. Produces mild acidity and tropical fruit notes. Leaves the beer with very little mouthfeel. See recipe recommendations for fermentation additions to boost mouthfeel. 90% + Attenuation. Low Flocculation. 60-75 F Temperature Range. High Alcohol Tolerance. | Isolated from a famous american wild ale brewery. |
GigaYeast
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis | Brussels Bruxellensis GB001 | Produces classic Brett “Barnyard” characteristics plus some subtle fruity aroma and moderate acidity. Adds a tart complexity to any beer. | |
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis | Tart Cherry Brett GB002 | Produces some Brett Barnyard funk plus stone fruit and cherry-like esters. This Strain also produces a moderate amount of acid that adds a tart complexity to the brew. | |
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis | Sweet Flemish Brett GB144 | Produces a sweet, slightly fruity profile with just a hint of barnyard and spicy phenolics |
Jasper Yeast LLC
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis "Chateaux" | JY87 | JY87 is a Brettanomyces yeast isolated by jasperyeast LLC. Fast-growing, it has almost Saccharomyces-like doubling times. Shows great fermentation as primary strain in a variety of beers. Ferments fast to 60% attenuation, after which fermentation slows down and more flavor and aroma is produced. Strong pineapple and stonefruit aroma after prolonged fermentations (3-9 months). Great companion to beers that could use some funk, and complements hoppy beers perfectly. Flocculation is low, strain will form a pellicle when oxygen is present. Sequencing of ITS regions indicated Brettanomyces bruxellensis. Micrograph of JY87 cells coming soon. | West-Flanders, Belgium. |
Omega Yeast Labs
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Claussenii | Dekkera anomala | B. anomala | Brettanomyces claussenii OYL-201 | Contributes more Brett aroma than flavor. Fruity, pineapple like aroma. Flocculation: low, Attenuation: 70-85%, Temp: >85°F, Alcohol Toelrance: medium-high, compares to WLP645. Pro brewers only. | |
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis | Brettanomyces bruxellensis OYL-202 | Medium intensity Brett character. Classic strain used in secondary fermentation for Belgian style beers and lambics. Flocculation: low, Attenuation: 70-85%, Temp: >85°F, Alcohol Tolerance: medium-high, compares to WLP650. Pro brewers only. | |
| Lambicus | Dekkera bruxellensis | B. lambicus | Brettanomyces lambicus OYL-203 | This strain has been described as producing horsey, smoky and spicy flavors. As the name suggests, this strain is found most often in Lambic style beers. Flocculation: low, Attenuation: 70-85%, Temp: >85°F, Alcohol Tolerance: medium-high, compares to WLP653. Pro brewers only. |
RVA Yeast Labs
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis | RVA 502 | A medium-intensity Brettanomyces yeast strain. Will add a bit of funk when added during the secondary. Typically used in Belgian-style beers, especially lambic. A famous Trappist brewery produces its unique beer with this yeast during secondary fermentation. | |
| Claussenii | Dekkera anomala | B. claussenii | RVA 501 | A low-intensity strain. Contributions from this strain are mostly aromas of pineapple and fruit. This strain prefers higher temperatures (85º F), but will produce nice aroma and subtle flavor at normal ale fermentation termperatures (68-72º F). | |
| Lambicus | Dekkera bruxellensis | B. lambicus | RVA 503 | High-intensity “Brett” strain. Very spicey with “smoky” and “horseblanket” flavors and aromas. This strain is used mostly in Lambics and Flanders sour beers. | |
| Unknown | Unknown | Unknown | RVA 804 | Produces some amazing aromas of pears, and other fruit esters. We highly recommend this strain for Belgian Dubbels. This strain also makes a very nice cider. A highly flocculating, medium-high attenuating strain adds nice complexity to stouts, and Belgian Ales and Specialty Belgian Ales. Flocculation: Medium, Attenuation: 78-85%, Suggested Temp Range: 65-72°F, Alcohol Tolerance: 14%. | This strain originates from local fruit trees. |
White Labs
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis | WLP650 | Barnyard | Not the same as WY's Brux |
| Bruxellensis | Dekkera bruxellensis | Brettanomyces bruxellensis Trois Vrai | WLP648 | Pear | The vrai (true, in French) Brettanomyces bruxellensis Trois. The infamous strain used for all-Brettanomyces fermentations, has a robust, complex sour character with aromas of pear. Best used as a primary fermentation strain. May be the same as BSI Drie? Profile is very similar to BSI Drie [69] [70]. |
| Claussenii | Dekkera anomala | B. claussenii | WLP645 | Fruity, pineapple | |
| Lambicus | Dekkera bruxellensis | B. lambicus | WLP653 | Horsey, Smoky, Spicy | Different from WY's "lambicus" |
Wyeast
| Common Name | Species Name | Synonym (Strain) Name | Lab/Package | Flavor/Aroma | Source Note |
|---|---|---|---|---|---|
| Anomalus | Dekkera anomala | B. anomalus | Wyeast | bottled stout - Burton on Trent, England. Discontinued. Cell count: 7.5 x 108 cells/mL [71]. | |
| Bruxellensis | Dekkera bruxellensis | B. bruxellensis | Wyeast 5112 | "sweaty horse blanket" | Not the same as WL's Brux. Cell count: 7.5 x 108 cells/mL [71]. |
| Claussennii | Dekkera anomala | B. claussenii | Wyeast 5151 | Notes of tropical fruit, pineapple and, to a lesser extent, peach and blueberry round out a classic Brett profile. Produces “horse blanket,” leathery, and smoky character, but at lower level than other Brett strains. Can be used as the primary strain for fermenting, but is often used after a primary fermentation with an S. cerevisiae strain, and in blends to produce sour beers. It is highly attenuative, given proper time to fully ferment out, and is known to create a pellicle during fermentation. | Private collection for Spring 2015. Cell count: 7.5 x 108 cells/mL [71]. |
| Lambicus | Dekkera bruxellensis | B. lambicus | Wyeast 5526 | Pie-cherry | Different from WL's "lambicus". Cell count: 7.5 x 108 cells/mL [71]. |
Smaller Labs
| Mfg | Taxonomy | Notes |
|---|---|---|
| BKYeast | Brett X1 | Suspected Brettanomyces Anomalus |
| BKYeast | Brett C1 | Isolate from Cantillon Iris |
| BKYeast | Brett C2 | Isolate from Cantillon Iris |
| BKYeast | Brett C3 | Isolate from Cantillon Iris |
| DCYeast | DCY01 | |
| Saccharolicious | Brett I | Brettanomyces yeast from a Walloon Trappist brewery that gives an earthy aroma to the beer. Recommended for secondary fermentation after primary fermentation with Trappist O. |
| Saccharolicious | Brett II | Fruity Brettanomyces yeast strain with an aroma that reminds of French cider. originates from Brasserie à Vapeur in Pipaix, Belgium, and was isolated from a bottle of Cochonne. |
Brett Blends (Brett only)
| Manufacturer | Package | Notes |
|---|---|---|
| East Coast Yeast | ECY34 Dirty Dozen Brett Blend | Twelve (12) different isolates of Brettanomyces exhibiting high production of barnyard "funk" and esters. Dryness, ripe fruit, and acidity will be encountered over a period of months and over time (>1 yr), may display gueuze-like qualities in complexity. Contains various isolates from lambic-producers, B. bruxellensis, B. anomala, B. lambicus, and B. naardenensis. For those who want the most from Brett yeast, whether a 100% Brett fermentation is desired or adding to secondary aging projects. Suggested fermentation temperature: 60-74 F. Attenuation high. |
| Omega Yeast Labs | All The Bretts | This will be an evolving blend comprised of nearly every Brettanomyces strain in our collection (inaugural release will contain 12 strains). When used in secondary, expect high attenuation and a fruity and funky complexity developing over time. Attenuation: 85+%; Flocculation: low; Temperature: 68F-85F. Homebrew pitch contains ~70 billion cells [72]. |
| The Yeast Bay | Beersel | Not overly funky but there is a sweaty note hanging behind lemon and citrus fruits, nice blend of subtle funk and citrus/fruit. All strains were identified as B. bruxellensis [73]. |
| The Yeast Bay | Brussels | Similar to Beersel but with more funk in aroma and less fruit, complex barnyard character. All strains were identified as B. bruxellensis [73]. |
| The Yeast Bay | Lochristi | Smells of Iris C2, probably the same, subtle blend with some delicate fruit, strawberry. All strains were identified as B. bruxellensis [73]. |
| The Yeast Bay | Amalgamation Brett Super Blend | 6 Brett blend to create a dry beer with a bright and complex fruit-forward flavor and aroma, accompanied by some funk. All strains were identified as B. bruxellensis [73]. |
Using Brett
Primary versus Secondary Fermentation
Brettanomyces can be pitched into a beer at many points in the beer's fermentation life cycle. If used as the primary fermenter, the beer that is produced is often fruit forward and not very funky. A large cell count will be needed (somewhere between an ale and lager pitching rate). See the 100% Brettanomyces Fermentation page for more information. If pitched into a beer that has already been fermented by Saccharomyces, a wider range of flavors including the funkier flavors can be produced (see the Brettanomyces Metabolism section above). A small cell count of Brettanomyces is plenty for creating these flavors, and normally a starter is not necessary. See the Mixed Fermentation and Funky Mixed Fermentations pages for more information on using Brett in secondary.
Starter Information
When pitching just Brettanomyces from a commercial pure or blended culture and no other microbes, it is recommended to make a starter for the culture. If the Brettanomyces is being pitched into secondary, no starter is necessary unless the brewer suspects that the Brettanomyces has lost a lot of viability due to age, heat exposure, etc., or prefers higher cell count pitches.
Two Approaches to Starters
There are generally two approaches to handling Brettanomyces starters. The first is to use a stir plate set to a medium-high RPM with tin foil on top of the flask for 7-8 days, cold crash for a few days, and then decant the beer before pitching the sedimented yeast. The second approach is to use an orbital shaker set to 80 RPM to create a semi-aerobic environment (this means that the oxygen levels are low, but also not non-existent) for 7-8 days as described in The Brettanomyces project [74], cold crashing can be skipped, and the entire starter is pitched into the wort. An alternative to the second approach is to use a stir plate on a very low setting so that only a very small "dimple" of a vortex is formed [75]. If a stir plate is not available, give the starter an initial dosage of pure O2, and then cover it with foil so that oxygen can slowly diffuse into the starter, and gently agitate as often as possible [76].
Oxygen levels are an important factor to consider when deciding which of the above two methods to use for a Brettanomyces starter. Brettanomyces creates acetic acid in the presence of oxygen, potentially leading to higher levels of ethyl acetate, which is considered an off flavor in higher amounts. As the amount of oxygen increases, cell growth increases, but so does acetic acid production. The amount of acetic acid produced is species/strain dependent, so some strains may benefit from more aeration without having the negative effect of creating too much acetic acid. Other strains may need a less aerobic starter (semi-aerobic) in order to produce the highest cell count with minimal acetic acid [77][78][79].
This presents a sort of "catch 22" when growing Brettanomyces in a starter. The brewer must weigh the pros and cons of how much aeration to provide. If the Brettanomyces is going to be used in a 100% Brettanomyces Fermentation, for example, then a stir plate may be the best choice. If the Brettanomyces is instead being pitched in secondary with the intention of long aging, then having a high cell count isn't as necessary and the risk of adding more acetic acid/ethyl acetate to an aging beer is greater. If a lot of acetic acid is produced during the starter, it is advised to cold crash and decant the starter. Brettanomyces can have a difficult time flocculating and settling out, even when cold crashed. The brewer may need to allow a few days for the cells to fully sediment [80]. Additionally, Brettanomyces that is cold crashed may be slower to begin fermentation. If the brewer believes that the amount of acetic acid produced was insignificant, then cold crashing can be skipped and the entire starter can be pitched.
Although more experiments and probably needed, agitation is believed to be an important factor for any species of microbe (yeast and bacteria). Gentle stirring on a stir plate or orbital shaker, or frequent gentle manual agitation leads to faster growth and a higher number of organisms. Agitation keeps the microbes in solution. It also maximizes the microbes' access to nutrients and disperses waste evenly. In a non-agitated starter, the microbes are limited to the diffusion rate of nutrients, leading to a slower and more stressful growth [81].
Maintaining a temperature of 70°-80°F/20°-26°C should be adequate for most strains. Brettanomyces cell growth typically takes about 7-8 days to reach it's maximum growth [82]. Thus, each step of a starter for Brett should be 7-8 days.
For more information regarding aeration and agitation effects on Brettanomyces growth, see Mark Trent's Brettanomyces Propagation Experiment.
Pitching Rate Calculators
Current yeast pitching calculators for brewers are not adequate for determining Brettanomyces pitching rates based on starter volume size because the maximum cell density of Brettanomyces per mL of wort is 3 to 6 times the cell density of Sacch. For example, a given Sacch strain may reach a cell density of 130 million cells per mL in a 1.040 wort (different Sacch strains can have different cell densities as well, although they are a lot lower than Brettanomyces overall). Different Brettanomyces strain cell densities have been reported to be 600 to 885 million cells per mL in 1.040 wort depending on the species/strain [82][83]. Since yeast calculators are based off of Sacch cell density, using one of these tools for Brettanomyces starters will create an unexpectedly high cell count in reality. There is not currently enough data to accurately determine starter volumes for Brettanomyces, particularly because each strain and species has a different maximum cell density per mL of wort. However, pitching around 500-600 mL of a Brettanomyces starter for 5 gallons of 1.060 SG wort will achieve a pitching rate that is similar to lager yeast pitching rates, which has been recommended for 100% Brettanomyces Fermentation. Omega Yeast Labs is currently working on a project to create a more accurate Brettanomyces pitching rate calculator (it will also contain pitching rate calculations for specific strains of Sacch, which is something that current yeast pitching calculators do not include) [83].
Given this information, many brewers historically have been using the lager pitching rate settings in online yeast pitching calculators for Brettanomyces starters (around 2000 mL, for example). Effectively, this means they have been pitching around 4 to 5 times the amount of Brettanomyces cells that they thought they were pitching. However, if this very high pitching rate is giving good results for brewers, it should continued to be used. Exploration of Brettanomyces pitching rates for 100% Brett fermentations is something to be desired once we know what our pitching rates actually are, and many brewers have been pitching 4-5 times the pitching rate for lagers if they use an online yeast pitching rate calculator instead of counting the cells under a microscope.
MYPG Growth Substrate
For yeast laboratories, "Malt Yeast Peptone Glucose" growth substrate has been shown to be a better substrate than wort for initially growing Brettanomyces from a plate or slant. When grown in wort, Brettanomyces will often go through a 24 hour lag phase, a growth phase, another lag phase, and a second growth phase (all within 7-8 days). When grown in MYPG substrate, there is only a single growth phase and no lag phase, which has been reported by Yakobson to produce a larger cell count in the same amount of time [84]. Cells grown in MYPG also are better adapted to grow in wort [85]. Practical instructions for making this substrate can be found on Jason Rodriguez's blog, "Brew Science - Homebrew Blog". Unfortunately, growing Brettanomyces pitches in MYPG for breweries isn't very practical due to needing almost 4 times the amount of MYPG versus wort to get the same pitching rate. In a brewery or homebrewery, using wort for Brettanomyces starters is more practical [86].
Example of a Homemade Orbital Shaker
Mark Trent's shaker platform (obtained from a used equipment outlet in Gilroy, CA called "Outback Equipment" ) used to create a semi-aerobic environment for Brettanomyces. Mark built an insulated box for it, and added temperature control. He can propagate up to 7 liters. This is running at 80 RPM as described in The Brettanomyces project [74][87].
Storing Brett
Long term storage of Brettanomyces should be frozen with glycerol, rather than agar plates or slants, which have been observed anecdotally to reduce viability of Brettanomyces over time [88][89]. Chad Yakobson noted that after storing Brettanomyces in a refrigerated environment (we don't know how Chad was storing the Brettanomyces cultures when he observed this, for example on agar plates or slants or something else.), after 6 months the Brettanomyces would die. If Brettanomyces is stored cold, it will be very sluggish and slow to start fermentation. Non-pure cultures (such as beer bottle dregs with Brettanomyces in it) should be stored refrigerated. Making a starter is highly recommended if the Brettanomyces culture has been stored cold [90].
Yakobson's observations were not scientifically quantified and details of his process are lacking (how was the Brettanomyces stored?), as far as we know. Richard Preiss of Escarpment Labs shared the results of a controlled experiment on MTF that showed that BSI's Brett brux Drie and WLP645 B. claussenii survived better in low ABV beer when stored at refrigeration temperatures rather than room temperatures, contradicting the anecdotal observations reported by Yakobson. The samples were grown in 1.040 DME wort until typical cell density was reached, and measured for >95% viability after growth with trypan blue stain and microscopy. 10ml samples of each were stored in sterile conical tubes for one month at different temperatures (4°C and 24°C). The samples were burped to avoid having head pressure as a variable. Trypan blue stain and microscopy were used to measure the viability after one month. After one month of storage at 4°C (39.2°F), the viability of B. claussenii was 92%, and BSI Drie was 72% viability. The samples stored for one month at 24°C (75.2°F) showed a significant drop in viability, with B. claussenii ending up at 40% and BSI Drie at 6% viability. This experiment also indicates that the viability of Brettanomyces strains/species after storage is strain/species dependent [91].
Mark Trent repeated Richard Preiss's experiment, but tested different mediums (wort, liquid MYPG, and water). Two Brettanomyces isolates, one from Orval and one from SARA Bernice, were grown in 10 degree Plato wort and MYPG. The isolates are labeled on the chart as "Brett O (Orval)" and "Brett T (Tim Clifford)" respectively. After growth was complete, 10 mL of aliquots were aseptically transferred to 15 mL centrifuge tubes. In addition, the Orval isolate was grown on a MYPG plate and 3 single colonies for each treatment were transferred to 1 mL of sterile RO water in a 2 mL glass tube. Each treatment was prepared in a duplicate and stored at either 22°C or 1°C. Viability was measured after 31 days. Data shown in the chart to the right. No other statistics were performed (there were no statistically significant differences between the different types of storage mediums at room temperature). All storage mediums shared results similar to Richard's results. This further shows that Brettanomyces survival is a function of temperature, with lower temperatures being beneficial towards survival [92].
Questions raised by MTF members in regards to these results:
- How does warm storage versus cold storage affect agar plates and slants?
- Are there any unexpected results if the samples are stored longer (Richard will update), and would this change if the Brettanomyces is periodically fed new sugars?
- How come using dregs from commercial beers stored at room temperature gives good results for brewers?
Tips From Brewers
The Yeast Bay Lochristi Brett Blend
- Both Ed Coffey and Amos Browne have noted that this blend ages particularly well.
- To make a starter for the Lochristi blend, run it semi-aerobic for 4-6 days in the 70's and then let it settle at room temp and decant what you can if the starter is large [93].
See Also
Additional Articles on MTF Wiki
- 100% Brettanomyces Fermentation
- Crooked Stave Artisan Beer Project
- Scientific Publications
- Secondary metabolites
- Mixed Cultures
- Mixed Fermentation
- Funky Mixed Fermentations
External Resources
- Family tree of Brettanomyces, by Eureka Brewing Blog.
- Insight into the Brettanomyces Mitochondrial Genome, by Eureka Brewing Blog.
- National Collection of Yeast Cultures in the UK - Database on what compounds different species/strains can ferment.
- The Brettanomyces Project - Chad Yakobon's Brett research.
- The Mad Fermentationist - Commercial Brettanomyces, Lactobacillus, and Pediococcus Descriptions
- The Mad Fermentationist - Comparison between English Ale yeast and Belgian Ale yeast primary fermentations, and Brett in secondary
- Blog Article on Brett and Glycosides by Cy Wood.
- Brettanomyces in Brewing the horse the goat and the barnyard, presentation by Chad Yakobson.
- Insight into the Dekkera anomala YV396 genome by Samuel Aeschlimann; self published on Eureka Brewing Blog.
- Esters - Table of esters and their smells.
References
- ↑ Wikipedia. Brettanomyces. Retrieved 2/24/2015.
- ↑ 2.0 2.1 2.2 2.3 The wine and beer yeast Dekkera bruxellensis. Anna Judith Schifferdecker, Sofia Dashko, Olena P. Ishchuk, and Jure Piškur. 7 July 201.
- ↑ Brettanomyces yeasts — From spoilage organisms to valuable contributors to industrial fermentations. Jan Steensels, Luk Daenen, Philippe Malcorps, Guy Derdelinckx, Hubert Verachtert, Kevin J. Verstrepen. International Journal of Food Microbiology Volume 206, 3 August 2015, Pages 24–38.
- ↑ Screening of yeast mycoflora in winery air samples and their risk of wine contamination. E. Ocón, P. Garijo, S. Sanz, C. Olarte, R. López, P. Santamaría, A.R. Gutiérrez. Food Control Volume 34, Issue 2, December 2013, Pages 261–267.
- ↑ 5.0 5.1 Yakobson, Chad. The Brettanomyces Project. Introduction. Retrieved 8/11/2015.
- ↑ Wines and Vines. New Research on Role of Yeast in Winemaking; report on a presentation by David Mills and Lucy Joseph from UC Davis. 11/14/2014. Retrieved 08/16/2015.
- ↑ Removal of Brettanomyces Bruxellensis from Red Wine Using Membrane Filtration. Umiker, Descenzo, Lee, and Edwards. 04/24/2012.
- ↑ Insight into the Dekkera anomala YV396 genome. Samuel Aeschlimann. Self published on Eureka Brewing Blog. Spet 2015.
- ↑ Daenen et al., 2008. Evaluation of the glycoside hydrolase activity of a Brettanomyces strain on glycosides from sour cherry (Prunus cerasus L.) used in the production of special fruit beers. FEMS Yeast Res. 8, 1103-1114.
- ↑ Wikipedia. Secondary Metabolite. Retrieved 6/2/2015.
- ↑ Yakobson, Chad. Pure Culture Fermentation Characteristics of Brettanomyces Yeast Species and Their Use in the Brewing Industry. Production of Secondary Metabolites. 2011.
- ↑ PubChem. Ethyl Acetate. Retrieved 08/15/2015.
- ↑ Ethyl Butyrate Beer Flavour Standard. FlavorActIV. Retrieved 6/20/2015.
- ↑ Flavoractiv. Ethyl butyrate. Retrieved 1/18/2015.
- ↑ 15.0 15.1 PubChem. Ethyl Butyrate. Retrieved 08/15/2015.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 16.8 16.9 Yakobson, Chad. Pure Culture Fermentation Characteristics of Brettanomyces Yeast Species and Their Use in the Brewing Industry. Pure Culture Fermentation Discussion. 2011.
- ↑ Encyclopedia of Food Microbiology. Batt, Carl A. Academic Press. Sep 28, 1999. Pg 320.
- ↑ Aroxa. ethyl hexanoate. Retrieved 1/18/2015.
- ↑ PubChem. Ethyl Caproate. Retrieved 08/15/2015.
- ↑ Chemspider. Ethylhexanoat. Retrieved 1/18/2015.
- ↑ Ethyl Octanoate. The Good Scents Company. Retrieved 5/28/2015.
- ↑ Chop & Brew - Episode 37: Influence of Mashing on Sour Beer Production by Michael Tonsmeire. NHC 2014 Presentation. At 26 minutes. Retrieved 5/28/2015.
- ↑ Esters Detection Tresholds & Molecular Structures. Leffingwell & Associates. Retrieved 5/28/2015.
- ↑ 24.0 24.1 PubChem. Ethyl Caprylate. Retrieved 08/15/2015.
- ↑ 25.0 25.1 Wikipedia. Ethyl Decanoate. Retrieved 1/18/2015.
- ↑ Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability. Ralf Günter Berger. Springer Science & Business Media, Mar 6, 2007. Pg 222.
- ↑ Spedding, Gary. Flavor notes for Michigan Craft Guild Conference. 2014.
- ↑ 28.0 28.1 28.2 28.3 Fenaroli's Handbook of Flavor Ingredients, Fifth Edition. George A. Burdock. CRC Press, Dec 3, 2004. Pg 587.
- ↑ 29.0 29.1 29.2 29.3 Supplemental Data for: Joseph, C.M.L., E.A. Albino, S.E. Ebeler, and L.F. Bisson. Brettanomyces bruxellensis aroma-active compounds determined by SPME GC-MS olfactory analysis. 2015.
- ↑ 30.0 30.1 Impact of Brettanomyces on Wine. Presentation by Lucy Joseph of UC Davis. Retrieved 08/15/2015.
- ↑ Best Aroma website. Ethyl Lactate. Retrieved 08/15/2015.
- ↑ Dictionary of Flavors. Dolf De Rovira. John Wiley & Sons, Feb 28, 2008. Pg 384.
- ↑ Haz-Map, Ethyl Lactate odor threshold.
- ↑ PubChem. Ethyl Lactate. Retrieved 08/15/2015.
- ↑ Yakobson, Chad. The Brettanomyces Project. Impact of the Initial Concentration of Lactic Acid on Pure Culture Fermentation. Retrieved 6/16/2015.
- ↑ 36.0 36.1 36.2 The Good Scents Company. Ethyl Valerate article. Retrieved 08/15/2015. Cite error: Invalid
<ref>tag; name "goodscents_ethylvalerate" defined multiple times with different content - ↑ 37.0 37.1 Fenaroli's Handbook of Flavor Ingredients, Fifth Edition. George A. Burdock. CRC Press, Dec 3, 2004. Pg 638.
- ↑ Organoleptic Threshold Values of Some Organic Acids in Beer. Sigmund Engan. 1973.
- ↑ Aroma of Beer, Wine and Distilled Alcoholic Beverages. L. Nykänen, H. Suomalainen. Springer Science & Business Media, May 31, 1983.
- ↑ Aroxa. Isaoamyl acetate. Retrieved 1/18/2015.
- ↑ PubChem. Isoamyl Acetate. Retrieved 08/25/2015.
- ↑ 42.0 42.1 Spaepen and Verachtert, 1982. Esterase Activity in the Genus Brettanomyces
- ↑ PubChem. Phenethyl Acetate. Retrieved 08/15/2015.
- ↑ YMDB. Phenethyl acetate.
- ↑ Burdock, George A. Fenaroli's Handbook of Flavor Ingredients, Fifth Edition. CRC Press. 2005. pg 1521.
- ↑ Characterization of aroma and flavor compounds present in lambic (gueuze) beer. Katherine A Thompson Witrick. 2012.
- ↑ Analysis of Growth Inhibition and Metabolism of Hydroxycinnamic Acids by Brewing and Spoilage Strains of Brettanomyces Yeast. Michael Lentz and Chad Harris. 2015.
- ↑ 48.0 48.1 48.2 48.3 48.4 48.5 Doss, Greg. Brettanomyces: Flavors and performance of single and multiple strain fermentations with respect to time. Presentation at 2008 NHC. pg 12.
- ↑ 49.0 49.1 49.2 49.3 49.4 49.5 Yakobson, Chad. Brettanomyces in Brewing the horse the goat and the barnyard. 1/14/2011
- ↑ Determination of 4-vinylgaiacol and 4-vinylphenol in top-fermented wheat beers by isocratic high performance liquid chromatography with ultraviolet detector. Mingguang Zhu; Yunqian Cui. Dec 2013.
- ↑ The Good Scents Company. 4-Vinylphenol. Retrieved 08/18/2015.
- ↑ 52.0 52.1 52.2 Molecular identification of Brettanomyces bruxellensis strains isolated from red wines and volatile phenol production. A. Oelofse, A. Lonvaud-Funel, M. du Toit. 2009.
- ↑ Aroxa Website. 4-Vinylguaiacol. Retrieved 08/19/2015.
- ↑ 54.0 54.1 The Good Scents Company. 2-methoxy-4-vinyl phenol. Retrieved 08/18/2015.
- ↑ Ferulic Acid Release and 4-Vinylguaiacol Formation during Brewing and Fermentation: Indications for Feruloyl Esterase Activity in Saccharomyces cerevisiae. Stefan Coghe, Koen Benoot, Filip Delvaux, Bart Vanderhaegen, and Freddy R. Delvaux. 2004.
- ↑ The biotransformation of simple phenolic compounds by Brettanomyces anomalus. Duncan A.N. Edlin1, Arjan Narbad, J. Richard Dickinson1 andDavid Lloyd. 2006.
- ↑ PubChem. 3-Vinylcatechol. Retrieved 08/18/2015.
- ↑ Aroxa Website. 4-Ethyl Phenol. Retrieved 08/19/2015.
- ↑ 59.0 59.1 Volatile Compounds in Foods and Beverages. Henk Maarse. CRC Press, Mar 29, 1991. Pg 514, 515.
- ↑ 60.0 60.1 PubChem Website. 4-Ethylphenol. Retrieved 08/19/2015.
- ↑ Aroxa Website. 4-Ethyl guaiacol. Retrieved 08/19/2015.
- ↑ 62.0 62.1 PubChem Website. 4-Ethyl-2-methoxyphenol. Retrieved 08/19/2015.
- ↑ 63.0 63.1 PubChem Website. 4-Ethylcatechol. Retrieved 08/19/2015.
- ↑ Botha, Janita J. Sensory, chemical and consumer analysis of Brettanomyces spoilage in South African wines. March 2010. Pg 2, 13, 17, 18
- ↑ Oelofse, Adriaan. Investigating the role of Brettanomyces and Dekkera during winemaking. December 2008.
- ↑ FlavorActV. Caprylic Acid. Retrieved 2/10/2015.
- ↑ "Decanoic acid". Wikipedia.
- ↑ Humbard, Matt. Milk The Funk Discussion. 3/10/2015.
- ↑ Ryan Steagall's conversation with White Labs on Facebook. April 10, 2015.
- ↑ White Labs 644 Explanation. 04/09/2015. Retrieved 5/2/2015.
- ↑ 71.0 71.1 71.2 71.3 Wyeast Specifications 2015 Retail Products. 2015.
- ↑ Conversation with Lance Shaner on MTF regarding Omega cell counts. 10/09/2015.
- ↑ 73.0 73.1 73.2 73.3 Conversation with Nick Impellitteri regarding taxonomy of the TYB Brett blends. 11/13/2015.
- ↑ 74.0 74.1 Yakobson, Chad. The Brettanomyces Project. Propagation and Batch Culture Methods. Retrieved 2/18/2015.
- ↑ Conversation with Mark Trent, Richard Preiss, and Roy Ventullo on MTF regarding creating a semi-aerobic starter without an orbital shaker. 11/06/2015
- ↑ Conversation with Nick Impellitteri on MTF in regards to semi-aerobic starters. 2/16/2015.
- ↑ Brettanomyces bruxellensis: effect of oxygen on growth and acetic acid production. Aguilar Uscanga, Délia1, and Strehaiano. 2003.
- ↑ Role of oxygen on acetic acid production by Brettanomyces/Dekkera in winemaking. Maurizio Ciani and Luisa Ferraro. April 1999.
- ↑ Acetic acid production by Dekkera/Brettanomyces yeasts. S.N. Feer. April 2002.
- ↑ Conversation with Richard Preiss of Escarpment Yeast Labs on MTF. 6/26/2015.
- ↑ Conversation with Bryan Heit about starters and agitation. 11/09/2015.
- ↑ 82.0 82.1 Yakobson, Chad. The Brettanomyces Project. Propagation and Batch Culture Results. Retrieved 2/17/2015
- ↑ 83.0 83.1 Conversation with Mark Trent and Lance Shaner on MTF regarding Brett pitching rates. 07-21-2015.
- ↑ Yakobson, Chad. The Brettanomyces Project. MYPG Compared to Wort as a Growth Substrate. Retrieved 2/18/2015.
- ↑ Yakobson, Chad. The Brettanomyces Project. Propagation and Batch Culture Discussion. Paragraph 5. Retrieved 2/18/2015.
- ↑ Conversation with Mark Trent, Lance Shaner, and Richard Preiss on MTF. 09/18/2015.
- ↑ Discussion with Mark Trent on Milk The Funk. 4/2/2015.
- ↑ Conversation with Matt Humbard, Ritchie Preiss, and Jeff Melo on MTF. 6/4/2015.
- ↑ Conversation with Nick Impellitteri on MTF regarding storing Brett on agar plates. 7/24/2015.
- ↑ Yakobson, Chad. Presentation at 2012 Music City Brew Off. At 43:00.
- ↑ Richard Preiss Brett storage experiment results on MTF. 7/24/2015.
- ↑ Conversation with Mark Trent on MTF. 09/10/2015.
- ↑ Nick Impellitteri of The Yeast Bay on a MTF thread. Feb 17, 2015.